Phosphorylation of cardiac troponin I at protein kinase C site threonine 144 depresses cooperative activation of thin filaments
- PMID: 20164197
- PMCID: PMC2852917
- DOI: 10.1074/jbc.M109.055657
Phosphorylation of cardiac troponin I at protein kinase C site threonine 144 depresses cooperative activation of thin filaments
Abstract
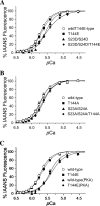
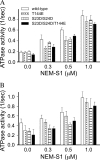
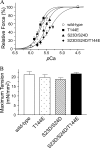
There is evidence for PKC-dependent multisite phosphorylation of cardiac troponin I (cTnI) at Ser-23 and Ser-24 (also PKA sites) in the cardiac-specific N-terminal extension and at Thr-144, a unique residue in the inhibitory region. The functional effect of these phosphorylations in combination is of interest in view of data indicating intramolecular interaction between the N-terminal extension and the inhibitory region of cTnI. To determine the role of PKC-dependent phosphorylation of cTnI on sarcomeric function, we measured contractile regulation at multiple levels of complexity. Ca(2+) binding to thin filaments reconstituted with either cTnI(wild-type) or pseudo-phosphorylated cTnI(S23D/S24D), cTnI(T144E), and cTnI(S23D/S24D/T144E) was determined. Compared with controls regulated by cTnI(wild-type), thin filaments with cTnI(S23D/S24D) and cTnI(S23D/S24D/T144E) exhibited decreased Ca(2+) sensitivity. In contrast, there was no significant difference between Ca(2+) binding to thin filaments with cTnI(wild-type) and with cTnI(T144E). Studies of the pCa-force relations in skinned papillary fibers regulated by these forms of cTnI yielded similar results. However, in both the Ca(2+) binding measurements and the skinned fiber tension measurements, the presence of cTnI(S23D/S24D/T144E) induced a much lower Hill coefficient than either wild type, S23D/S24D, or T144E. These data highlight the importance of thin filament-based cooperative mechanisms in cardiac regulation, with implications for mechanisms of control of function in normal and pathological hearts.
Figures


Similar articles
-
Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity.J Biol Chem. 2003 Mar 28;278(13):11265-72. doi: 10.1074/jbc.M210712200. Epub 2003 Jan 27. J Biol Chem. 2003. PMID: 12551921
-
PKA phosphorylation of cardiac troponin I modulates activation and relaxation kinetics of ventricular myofibrils.Biophys J. 2014 Sep 2;107(5):1196-1204. doi: 10.1016/j.bpj.2014.07.027. Biophys J. 2014. PMID: 25185555 Free PMC article.
-
Restrictive Cardiomyopathy Troponin I R145W Mutation Does Not Perturb Myofilament Length-dependent Activation in Human Cardiac Sarcomeres.J Biol Chem. 2016 Oct 7;291(41):21817-21828. doi: 10.1074/jbc.M116.746172. Epub 2016 Aug 24. J Biol Chem. 2016. PMID: 27557662 Free PMC article.
-
Regulation of cardiac contractile function by troponin I phosphorylation.Cardiovasc Res. 2005 Apr 1;66(1):12-21. doi: 10.1016/j.cardiores.2004.12.022. Cardiovasc Res. 2005. PMID: 15769444 Review.
-
The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation.Biochem Biophys Res Commun. 2008 Apr 25;369(1):82-7. doi: 10.1016/j.bbrc.2007.12.114. Epub 2007 Dec 26. Biochem Biophys Res Commun. 2008. PMID: 18162178 Free PMC article. Review.
Cited by
-
Tropomyosin dephosphorylation results in compensated cardiac hypertrophy.J Biol Chem. 2012 Dec 28;287(53):44478-89. doi: 10.1074/jbc.M112.402040. Epub 2012 Nov 12. J Biol Chem. 2012. PMID: 23148217 Free PMC article.
-
Troponin I modulation of cardiac performance: Plasticity in the survival switch.Arch Biochem Biophys. 2019 Mar 30;664:9-14. doi: 10.1016/j.abb.2019.01.025. Epub 2019 Jan 23. Arch Biochem Biophys. 2019. PMID: 30684464 Free PMC article. Review.
-
Troponin I - a comprehensive review of its function, structure, evolution, and role in muscle diseases.Anim Cells Syst (Seoul). 2025 Jul 28;29(1):446-468. doi: 10.1080/19768354.2025.2533821. eCollection 2025. Anim Cells Syst (Seoul). 2025. PMID: 40735528 Free PMC article. Review.
-
Inherited cardiomyopathies caused by troponin mutations.J Geriatr Cardiol. 2013 Mar;10(1):91-101. doi: 10.3969/j.issn.1671-5411.2013.01.014. J Geriatr Cardiol. 2013. PMID: 23610579 Free PMC article.
-
Cardiac troponin structure-function and the influence of hypertrophic cardiomyopathy associated mutations on modulation of contractility.Arch Biochem Biophys. 2016 Jul 1;601:11-21. doi: 10.1016/j.abb.2016.02.004. Epub 2016 Feb 4. Arch Biochem Biophys. 2016. PMID: 26851561 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

