Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations
- PMID: 20164212
- PMCID: PMC2857991
- DOI: 10.1530/EJE-09-0861
Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations
Abstract
Objective: The phenotype associated with heterozygous HNF4A gene mutations has recently been extended to include diazoxide responsive neonatal hypoglycemia in addition to maturity-onset diabetes of the young (MODY). To date, mutation screening has been limited to patients with a family history consistent with MODY. In this study, we investigated the prevalence of HNF4A mutations in a large cohort of patients with diazoxide responsive hyperinsulinemic hypoglycemia (HH).
Subjects and methods: We sequenced the ABCC8, KCNJ11, GCK, GLUD1, and/or HNF4A genes in 220 patients with HH responsive to diazoxide. The order of genetic testing was dependent upon the clinical phenotype.
Results: A genetic diagnosis was possible for 59/220 (27%) patients. K(ATP) channel mutations were most common (15%) followed by GLUD1 mutations causing hyperinsulinism with hyperammonemia (5.9%), and HNF4A mutations (5%). Seven of the 11 probands with a heterozygous HNF4A mutation did not have a parent affected with diabetes, and four de novo mutations were confirmed. These patients were diagnosed with HI within the first week of life (median age 1 day), and they had increased birth weight (median +2.4 SDS). The duration of diazoxide treatment ranged from 3 months to ongoing at 8 years.
Conclusions: In this large series, HNF4A mutations are the third most common cause of diazoxide responsive HH. We recommend that HNF4A sequencing is considered in all patients with diazoxide responsive HH diagnosed in the first week of life irrespective of a family history of diabetes, once K(ATP) channel mutations have been excluded.
Figures
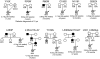
References
-
- Kapoor RR, Flanagan SE, James C, Shield J, Ellard S, Hussain K. Hypoglycaemia hypoglycaemia. Archives of Disease in Childhood. 2009;94:450–457. - PubMed
-
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, Aguilar-Bryan L, Gagel RF, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. - PubMed
-
- Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Human Molecular Genetics. 1996;5:1809–1812. - PubMed
-
- Taschenberger G, Mougey A, Shen S, Lester LB, LaFranchi S, Shyng SL. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. Journal of Biological Chemistry. 2002;277:17139–17146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

