Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate
- PMID: 20164221
- PMCID: PMC2863772
- DOI: 10.1128/JVI.02670-09
Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate
Abstract
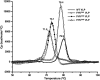
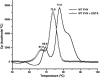
Divalent metal ions are components of numerous icosahedral virus capsids. Flock House virus (FHV), a small RNA virus of the family Nodaviridae, was utilized as an accessible model system with which to address the effects of metal ions on capsid structure and on the biology of virus-host interactions. Mutations at the calcium-binding sites affected FHV capsid stability and drastically reduced virus infectivity, without altering the overall architecture of the capsid. The mutations also altered the conformation of gamma, a membrane-disrupting, virus-encoded peptide usually sequestered inside the capsid, by increasing its exposure under neutral pH conditions. Our data demonstrate that calcium binding is essential for maintaining a pH-based control on gamma exposure and host membrane disruption, and they reveal a novel rationale for the metal ion requirement during virus entry and infectivity. In the light of the phenotypes displayed by a calcium site mutant of FHV, we suggest that this mutant corresponds to an early entry intermediate formed in the endosomal pathway.
Figures



Similar articles
-
Dissecting the functional domains of a nonenveloped virus membrane penetration peptide.J Virol. 2009 Jul;83(13):6929-33. doi: 10.1128/JVI.02299-08. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369344 Free PMC article.
-
Rescue of maturation-defective flock house virus infectivity with noninfectious, mature, viruslike particles.J Virol. 2008 Feb;82(4):2025-7. doi: 10.1128/JVI.02278-07. Epub 2007 Dec 12. J Virol. 2008. PMID: 18077727 Free PMC article.
-
Low endocytic pH and capsid protein autocleavage are critical components of Flock House virus cell entry.J Virol. 2009 Sep;83(17):8628-37. doi: 10.1128/JVI.00873-09. Epub 2009 Jun 24. J Virol. 2009. PMID: 19553341 Free PMC article.
-
Flock house virus: a model system for understanding non-enveloped virus entry and membrane penetration.Curr Top Microbiol Immunol. 2010;343:1-22. doi: 10.1007/82_2010_35. Curr Top Microbiol Immunol. 2010. PMID: 20407886 Review.
-
Recent insights into the biology and biomedical applications of Flock House virus.Cell Mol Life Sci. 2008 Sep;65(17):2675-87. doi: 10.1007/s00018-008-8037-y. Cell Mol Life Sci. 2008. PMID: 18516498 Free PMC article. Review.
Cited by
-
The SARS-CoV-2 Hydra, a tiny monster from the 21st century: Thermodynamics of the BA.5.2 and BF.7 variants.Microb Risk Anal. 2023 Apr;23:100249. doi: 10.1016/j.mran.2023.100249. Epub 2023 Feb 4. Microb Risk Anal. 2023. PMID: 36777924 Free PMC article.
-
Biothermodynamics of Viruses from Absolute Zero (1950) to Virothermodynamics (2022).Vaccines (Basel). 2022 Dec 9;10(12):2112. doi: 10.3390/vaccines10122112. Vaccines (Basel). 2022. PMID: 36560522 Free PMC article. Review.
-
Nucleic acid packaging in viruses.Curr Opin Struct Biol. 2012 Feb;22(1):65-71. doi: 10.1016/j.sbi.2011.11.002. Epub 2012 Jan 23. Curr Opin Struct Biol. 2012. PMID: 22277169 Free PMC article. Review.
-
Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection.PLoS Pathog. 2015 Oct 22;11(10):e1005203. doi: 10.1371/journal.ppat.1005203. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26491970 Free PMC article.
-
Uncoating Mechanism of Carnation Mottle Virus Revealed by Cryo-EM Single Particle Analysis.Sci Rep. 2015 Oct 7;5:14825. doi: 10.1038/srep14825. Sci Rep. 2015. PMID: 26442593 Free PMC article.
References
-
- Adamec, T., Z. Palkova, K. Velkova, J. Stokrova, and J. Forstova. 2005. Point mutation in calcium-binding domain of mouse polyomavirus VP1 protein does not prevent virus-like particle formation, but changes VP1 interactions with Saccharomyces cerevisiae cell structures. FEMS Yeast Res. 5:331-340. - PubMed
-
- Aramayo, R., C. Merigoux, E. Larquet, P. Bron, J. Perez, C. Dumas, P. Vachette, and N. Boisset. 2005. Divalent ion-dependent swelling of tomato bushy stunt virus: a multi-approach study. Biochim. Biophys. Acta 1724:345-354. - PubMed
-
- Arias-Moreno, X., A. Velazquez-Campoy, J. C. Rodriguez, M. Pocovi, and J. Sancho. 2008. Mechanism of low density lipoprotein (LDL) release in the endosome: implications of the stability and Ca2+ affinity of the fifth binding module of the LDL receptor. J. Biol. Chem. 283:22670-22679. - PubMed
-
- Banerjee, M., and J. E. Johnson. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept. Sci. 9:16-27. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

