Rotavirus differentially infects and polyclonally stimulates human B cells depending on their differentiation state and tissue of origin
- PMID: 20164228
- PMCID: PMC2863723
- DOI: 10.1128/JVI.02550-09
Rotavirus differentially infects and polyclonally stimulates human B cells depending on their differentiation state and tissue of origin
Abstract
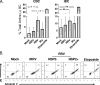
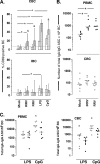
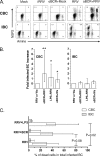
We have shown previously that rotavirus (RV) can infect murine intestinal B220(+) cells in vivo (M. Fenaux, M. A. Cuadras, N. Feng, M. Jaimes, and H. B. Greenberg, J. Virol. 80:5219-5232, 2006) and human blood B cells in vitro (M. C. Mesa, L. S. Rodriguez, M. A. Franco, and J. Angel, Virology 366:174-184, 2007). However, the effect of RV on B cells, especially those present in the human intestine, the primary site of RV infection, is unknown. Here, we compared the effects of the in vitro RV infection of human circulating (CBC) and intestinal B cells (IBC). RV infected four times more IBC than CBC, and in both types of B cells the viral replication was highly restricted to the memory subset. RV induced cell death in 30 and 3% of infected CBC and IBC, respectively. Moreover, RV induced activation and differentiation into antibody-secreting cells (ASC) of CBC but not IBC when the B cells were present with other mononuclear cells. However, RV did not induce these effects in purified CBC or IBC, suggesting the participation of other cells in activating and differentiating CBC. RV infection was associated with enhanced interleukin-6 (IL-6) production by CBC independent of viral replication. The infection of the anti-B-cell receptor, lipopolysaccharide, or CpG-stimulated CBC reduced the secretion of IL-6 and IL-8 and decreased the number of ASC. These inhibitory effects were associated with an increase in viral replication and cell death and were observed in polyclonally stimulated CBC but not in IBC. Thus, RV differentially interacts with primary human B cells depending on their tissue of origin and differentiation stage, and it affects their capacity to modulate the local and systemic immune responses.
Figures



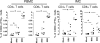
Similar articles
-
Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model.Gut Microbes. 2014;5(5):639-51. doi: 10.4161/19490976.2014.969972. Gut Microbes. 2014. PMID: 25483333 Free PMC article.
-
Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response.J Immunol. 2002 Mar 1;168(5):2173-81. doi: 10.4049/jimmunol.168.5.2173. J Immunol. 2002. PMID: 11859103
-
Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production.J Immunol. 2001 Feb 1;166(3):1894-902. doi: 10.4049/jimmunol.166.3.1894. J Immunol. 2001. PMID: 11160237
-
The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7.J Immunol. 1998 Oct 15;161(8):4227-35. J Immunol. 1998. PMID: 9780197
-
Correlates of protection for rotavirus vaccines: Possible alternative trial endpoints, opportunities, and challenges.Hum Vaccin Immunother. 2014;10(12):3659-71. doi: 10.4161/hv.34361. Hum Vaccin Immunother. 2014. PMID: 25483685 Free PMC article. Review.
Cited by
-
Rotavirus structural proteins and dsRNA are required for the human primary plasmacytoid dendritic cell IFNalpha response.PLoS Pathog. 2010 Jun 3;6(6):e1000931. doi: 10.1371/journal.ppat.1000931. PLoS Pathog. 2010. PMID: 20532161 Free PMC article.
-
Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model.Gut Microbes. 2014;5(5):639-51. doi: 10.4161/19490976.2014.969972. Gut Microbes. 2014. PMID: 25483333 Free PMC article.
-
Comparative In Vitro and In Vivo Studies of Porcine Rotavirus G9P[13] and Human Rotavirus Wa G1P[8].J Virol. 2015 Oct 14;90(1):142-51. doi: 10.1128/JVI.02401-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468523 Free PMC article.
-
Total and Envelope Protein-Specific Antibody-Secreting Cell Response in Pediatric Dengue Is Highly Modulated by Age and Subsequent Infections.PLoS One. 2016 Aug 25;11(8):e0161795. doi: 10.1371/journal.pone.0161795. eCollection 2016. PLoS One. 2016. PMID: 27560782 Free PMC article.
-
A working model of how noroviruses infect the intestine.PLoS Pathog. 2015 Feb 27;11(2):e1004626. doi: 10.1371/journal.ppat.1004626. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25723501 Free PMC article. No abstract available.
References
-
- Anderton, E., J. Yee, P. Smith, T. Crook, R. E. White, and M. J. Allday. 2008. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt's lymphoma. Oncogene 27:421-433. - PubMed
-
- Angel, J., M. A. Franco, and H. B. Greenberg. 2007. Rotavirus vaccines: recent developments and future considerations. Nat. Rev. Microbiol. 5:529-539. - PubMed
-
- Baccam, M., S. Y. Woo, C. Vinson, and G. A. Bishop. 2003. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-kappa B, AP-1, and C/EBP. J. Immunol. 170:3099-3108. - PubMed
-
- Bargatze, R. F., M. A. Jutila, and E. C. Butcher. 1995. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3:99-108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

