Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults
- PMID: 20164251
- PMCID: PMC2849330
- DOI: 10.1128/CVI.00436-09
Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults
Abstract
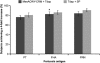
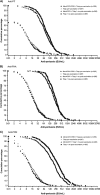
This study evaluated the safety, tolerability, and immunogenicity of an investigational quadrivalent meningococcal conjugate vaccine, MenACWY-CRM, when administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis (Tdap) vaccine, in subjects aged 11 to 25 years. Subjects received either MenACWY-CRM and Tdap, MenACWY-CRM and saline placebo, or Tdap and saline placebo. No significant increase in reactogenicity and no clinically significant vaccine-related adverse events (AEs) occurred when MenACWY-CRM and Tdap were administered concomitantly. Similar immunogenic responses to diphtheria, tetanus, and meningococcal (serogroups A, C, W-135, and Y) antigens were observed, regardless of concomitant vaccine administration. Antipertussis antibody responses were comparable between vaccine groups for filamentous hemagglutinin and were slightly lower, although not clinically significantly, for pertussis toxoid and pertactin when the two vaccines were administered concomitantly. These results indicate that the investigational MenACWY-CRM vaccine is well tolerated and immunogenic and that it can be coadministered with Tdap to adolescents and young adults.
Figures
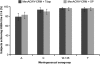

References
-
- American Academy of Pediatrics Committee on Infectious Diseases. 2005. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 116:496-505. - PubMed
-
- Bilukha, O. O., N. Rosenstein, et al. 2005. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 54(RR-7):1-21. - PubMed
-
- Broder, K. R., M. M. Cortese, J. K. Iskander, K. Kretsinger, B. A. Slade, K. H. Brown, C. M. Mijalski, T. Tiwari, E. J. Weston, A. C. Chon, P. U. Srivastava, J. S. Moran, B. Schwartz, and T. V. Murphy. 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 55(RR-3):1-34. - PubMed
-
- Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thorton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwight, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. - PMC - PubMed
-
- Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

