A DNA vaccine-encoded nucleoprotein of influenza virus fails to induce cellular immune responses in a diabetic mouse model
- PMID: 20164252
- PMCID: PMC2849332
- DOI: 10.1128/CVI.00445-09
A DNA vaccine-encoded nucleoprotein of influenza virus fails to induce cellular immune responses in a diabetic mouse model
Abstract
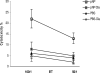
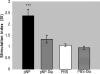
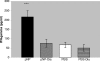
Influenza virus infections cause yearly epidemics and are a major cause of lower respiratory tract illnesses in humans worldwide. Influenza virus has long been recognized to be associated with higher morbidity and mortality in diabetic patients. Vaccination is an effective tool to prevent influenza virus infection in this group of patients. Vaccines employing recombinant-DNA technologies are an alternative to inactivated virus and live attenuated virus vaccines. Internal highly conserved viral nucleoprotein (NP) can be delivered as a DNA vaccine to provide heterosubtypic immunity, offering resistance against various influenza virus strains. In this study, we investigated the efficacy of an NP DNA vaccine for induction of cell-mediated immune responses and protection against influenza virus infection in a mouse model of diabetes. Healthy and diabetic BALB/c mice were immunized on days 0, 14, and 28 by injection of NP DNA vaccine. Two weeks after the last immunization, the cellular immune response was evaluated by gamma interferon (IFN-gamma), lymphocyte proliferation, and cytotoxicity assays. The mice were challenged with influenza virus, and the viral titers in the lungs were measured on day 4. Diabetic mice showed significantly smaller amounts of IFN-gamma production, lymphocyte proliferation, and cytotoxicity responses than nondiabetic mice. Furthermore, higher titers of the influenza virus were detected after challenge in the lungs of the diabetic mice. The present data suggest that the NP DNA vaccine with the protocol of immunization described here is not able to induce efficient cellular immune responses against influenza virus infection in diabetic mice.
Figures


References
-
- Calvet, H. M., and T. T. Yoshikawa. 2001. Infection in diabetes. Infect. Dis. Clin. North Am. 15:407-421. - PubMed
-
- Carrat, F., and A. Flahault. 2007. Influenza vaccine: the challenge of antigenic drift. Vaccine 25:6852-6862. - PubMed
-
- Diepersloot, R. J., K. P. Bouter, W. E. Beyer, J. B. Hoekstra, and N. Masurel. 1987. Humoral immune response and delayed type hypersensitivity to influenza vaccine in patients with diabetes mellitus. Diabetologia 30:397-401. - PubMed
-
- Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. - PubMed
-
- Drape, R. J., M. D. Macklin, L. J. Barr, S. Jones, J. R. Haynes, and H. J. Dean. 2006. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 24:4475-4481. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

