Performance evaluation of the new Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test version 2.0 for quantification of human immunodeficiency virus type 1 RNA
- PMID: 20164281
- PMCID: PMC2849552
- DOI: 10.1128/JCM.01832-09
Performance evaluation of the new Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test version 2.0 for quantification of human immunodeficiency virus type 1 RNA
Abstract
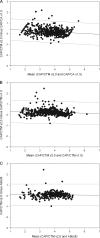
Despite FDA approval and CE marking of commercial tests, manufacturer-independent testing of the technical aspects of newly developed tests is important. To evaluate the analytical performance and explore the clinical applicability of the new Roche COBAS AmpliPrep COBAS TaqMan HIV-1 test, version 2.0 (CAP/CTM v2.0), platform comparison was performed with the Roche CAP/CTM test, version 2.0, the COBAS Amplicor HIV-1 Monitor Test, version 1.5 (CAP/CA v1.5), the COBAS AmpliPrep COBAS TaqMan HIV-1 Test (CAP/CTM v1.0), and the Abbott m2000 RealTime HIV-1 assay on panels and diagnostic samples. Specificity was tested for HIV-2 samples. Furthermore, samples from HIV-1-seropositive individuals with CAP/CA v1.5-measured viral loads below 50 HIV-1 RNA copies per ml (cp/ml) and replicates of HIV-1-seronegative plasma were tested in a checkerboard analysis. CAP/CTM v2.0 is HIV-1 specific, with broad genotype inclusivity and no serious underquantification of viral load relative to the other assays used. Low viral loads below the threshold of quantification for CAP/CA v1.5 are observed with CAP/CTM v2.0. A CAP/CTM v2.0-measured viral load of >50 copies/ml in these samples correlated with therapy failure. In conclusion, CAP/CTM v2.0 is an accurate and reliable test for HIV-1 viral load measurement relative to the other assays used with respect to specificity, sensitivity, and genotype inclusivity.
Figures

References
-
- Abbott, M. A., B. J. Poiesz, B. C. Byrne, S. Kwok, J. J. Sninsky, and G. D. Ehrlich. 1988. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J. Infect. Dis. 158:1158-1169. - PubMed
-
- Aberg, J. A., J. E. Kaplan, H. Libman, P. Emmanuel, J. R. Anderson, V. E. Stone, J. M. Oleske, J. S. Currier, and J. E. Gallant. 2009. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 49:651-681. - PubMed
-
- Compton, J. 1991. Nucleic acid sequence-based amplification. Nature 350:91-92. - PubMed
-
- de Baar, M. P., M. W. van Dooren, E. de Rooij, M. Bakker, B. van Gemen, J. Goudsmit, and A. de Ronde. 2001. Single rapid real-time monitored isothermal RNA amplification assay for quantification of human immunodeficiency virus type 1 isolates from groups M, N, and O. J. Clin. Microbiol. 39:1378-1384. - PMC - PubMed
-
- Dewar, R. L., H. C. Highbarger, M. D. Sarmiento, J. A. Todd, M. B. Vasudevachari, R. T. Davey, Jr., J. A. Kovacs, N. P. Salzman, H. C. Lane, and M. S. Urdea. 1994. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J. Infect. Dis. 170:1172-1179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

