Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis
- PMID: 20164296
- PMCID: PMC2853981
- DOI: 10.1210/jc.2009-1703
Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis
Abstract
Context: Teriparatide increases both bone formation and bone resorption.
Objective: We sought to determine whether combining teriparatide with an antiresorptive agent would alter its anabolic action.
Design and setting: This was a randomized controlled trial conducted in a single university hospital.
Patients and intervention: We randomized 93 postmenopausal women with low bone mineral density (BMD) to alendronate 10 mg daily (group 1), teriparatide 40 microg sc daily (group 2), or both (group 3) for 30 months. Teriparatide was begun at month 6.
Main outcome measures: BMD of the lumbar spine, proximal femur, proximal radius, and total body was measured by dual-energy x-ray absorptiometry (DXA) every 6 months. Lumbar spine trabecular BMD was measured at baseline and month 30 by quantitative computed tomography. Serum osteocalcin, N-terminal propeptide of type 1 collagen, and N-telopeptide levels were assessed frequently. Women who had at least one repeat DXA scan on therapy were included in the analyses (n = 69).
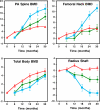
Results: DXA spine BMD increased more in women treated with teriparatide alone than with alendronate alone (18 +/- 11 vs. 7 +/- 4%; P < 0.001) or both (18+/-11 vs. 12 +/- 9%; P = 0.045). Similarly, femoral neck BMD increased more in women treated with teriparatide alone than with alendronate alone (11 +/- 5 vs. 4 +/- 4%; P < 0.001) or both (11 +/- 5 vs. 3 +/- 5%; P < 0.001). Quantitative computed tomography spine BMD increased 1 +/- 7, 61 +/- 31, and 24 +/- 24% in groups 1, 2, and 3 (P < 0.001 for all comparisons). Serum osteocalcin, N-terminal propeptide of type 1 collagen, and cross-linked N-telopeptides of type I collagen increased more with teriparatide alone than with both (P < 0.001 for each marker).
Conclusion: Alendronate reduces the ability of teriparatide to increase BMD and bone turnover in women.
Figures

References
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE 1996 Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541 - PubMed
-
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ 1998 Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. Results from the Fracture Intervention Trial. JAMA 280:2077–2082 - PubMed
-
- Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM 1998 Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized, controlled trial. JAMA 280:1067–1073 - PubMed
-
- Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM 1994 Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med 331:1618–1623 - PubMed
-
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs Jr RW, Dequeker J, Favus M, The Alendronate Phase III Osteoporosis Treatment Study Group 1995 Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443 - PubMed

