Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8
- PMID: 20164300
- PMCID: PMC2872957
- DOI: 10.1124/jpet.109.165209
Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8
Abstract
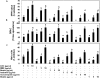
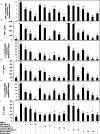
Activation of mast cells in rheumatoid synovial tissue has often been associated with tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and IL-8 production and disease pathogenesis by adjacent cell types. Butea monosperma (BM) is a well known medicinal plant in India and the tropics. The aim of this study was to examine whether a standardized extract of BM flower (BME) could inhibit inflammatory reactions in human mast cells (HMC) using activated HMC-1 cells as a model. Four previously characterized polyphenols--butrin, isobutrin, isocoreopsin, and butein--were isolated from BME by preparative thin layer chromatography, and their purity and molecular weights were determined by liquid chromatography/mass spectrometry analysis. Our results showed that butrin, isobutrin, and butein significantly reduced the phorbol 12-myristate 13-acetate and calcium ionophore A23187-induced inflammatory gene expression and production of TNF-alpha, IL-6, and IL-8 in HMC-1 cells by inhibiting the activation of NF-kappaB. In addition, isobutrin was most potent in suppressing the NF-kappaB p65 activation by inhibiting IkappaBalpha degradation, whereas butrin and butein were relatively less effective. In vitro kinase activity assay revealed that isobutrin was a potent inhibitor of IkappaB kinase complex activity. This is the first report identifying the molecular basis of the reported anti-inflammatory effects of BME and its constituents butrin, isobutrin, and butein. The novel pharmacological actions of these polyphenolic compounds indicate potential therapeutic value for the treatment of inflammatory and other diseases in which activated mast cells play a role.
Figures


Similar articles
-
Protective effect of a Butea monosperma (Lam.) Taub. flowers extract against skin inflammation: antioxidant, anti-inflammatory and matrix metalloproteinases inhibitory activities.J Ethnopharmacol. 2013 Jul 9;148(2):537-43. doi: 10.1016/j.jep.2013.05.001. Epub 2013 May 13. J Ethnopharmacol. 2013. PMID: 23680157
-
Cheongyeolsaseuptang inhibits production of TNF-alpha, IL-6 and IL-8 as well as NF-kappa B activation in human mast cells.J Ethnopharmacol. 2005 Feb 10;97(1):83-8. doi: 10.1016/j.jep.2004.10.018. Epub 2004 Dec 10. J Ethnopharmacol. 2005. PMID: 15652280
-
Anti-inflammatory effect of Poncirus trifoliata fruit through inhibition of NF-kappaB activation in mast cells.Toxicol In Vitro. 2006 Oct;20(7):1071-6. doi: 10.1016/j.tiv.2006.02.003. Epub 2006 Mar 6. Toxicol In Vitro. 2006. PMID: 16574371
-
Butea monosperma as a collective phytomedicine and environmentally sustainable, conservative, and beneficial plant.Arch Razi Inst. 2024 Jun 30;79(3):465-474. doi: 10.32592/ARI.2024.79.3.465. eCollection 2024 Jun. Arch Razi Inst. 2024. PMID: 39736959 Free PMC article. Review.
-
The role of chalcones in suppression of NF-κB-mediated inflammation and cancer.Int Immunopharmacol. 2011 Mar;11(3):295-309. doi: 10.1016/j.intimp.2010.12.006. Epub 2010 Dec 22. Int Immunopharmacol. 2011. PMID: 21184860 Free PMC article. Review.
Cited by
-
Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes.Arthritis Res Ther. 2010;12(5):R195. doi: 10.1186/ar3166. Epub 2010 Oct 18. Arthritis Res Ther. 2010. PMID: 20955562 Free PMC article.
-
Silver Citrate Nanoparticles Inhibit PMA-Induced TNFα Expression via Deactivation of NF-κB Activity in Human Cancer Cell-Lines, MCF-7.Int J Nanomedicine. 2020 Oct 30;15:8479-8493. doi: 10.2147/IJN.S274098. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33154638 Free PMC article.
-
Mitochondrial Role in Intrinsic Apoptosis Induced by a New Synthesized Chalcone in Hepatocellular Carcinoma Cells.Biomedicines. 2022 Dec 2;10(12):3120. doi: 10.3390/biomedicines10123120. Biomedicines. 2022. PMID: 36551876 Free PMC article.
-
Effect of Butein, a Plant Polyphenol, on Apoptosis and Necroptosis of Prostate Cancer Cells in 2D and 3D Cultures.Life (Basel). 2025 May 22;15(6):836. doi: 10.3390/life15060836. Life (Basel). 2025. PMID: 40566490 Free PMC article.
-
Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes.J Cell Mol Med. 2016 Dec;20(12):2241-2248. doi: 10.1111/jcmm.12897. Epub 2016 Aug 12. J Cell Mol Med. 2016. PMID: 27515563 Free PMC article.
References
-
- Arend WP, Dayer JM. (1995) Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum 38:151–160 - PubMed
-
- Azzolina A, Bongiovanni A, Lampiasi N. (2003) Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta 1643:75–83 - PubMed
-
- Bandara BMR, Kumar NS, Wimalasiri KMS. (1990) Constituents of the stem bark from Butea monosperma. J Natl Sci Counc Sri Lanka 18:97–103
-
- Bhargava SK. (1986) Estrogenic and postcoital anticonceptive activity in rats of butin isolated from Butea monosperma seed. J Ethnopharmacology 18:95–101 - PubMed
-
- Blois MS. (1958) Antioxidant determination by the use of a stable free radical. Nature 181:1199–1200
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

