Hormonal modulation of sensorimotor integration
- PMID: 20164325
- PMCID: PMC2840715
- DOI: 10.1523/JNEUROSCI.5533-09.2010
Hormonal modulation of sensorimotor integration
Abstract
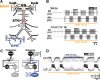
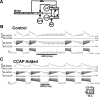
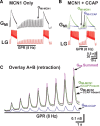
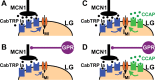
Neuronal circuits commonly receive simultaneous inputs from descending, ascending, and hormonal systems. Thus far, however, most such inputs have been studied individually to determine their influence on a given circuit. Here, we examine the integrated action of the hormone crustacean cardioactive peptide (CCAP) and the gastropyloric receptor (GPR) proprioceptor neuron on the biphasic gastric mill (chewing) rhythm driven by the projection neuron modulatory commissural neuron 1 (MCN1) in the isolated crab stomatogastric ganglion. In control saline, GPR stimulation selectively prolongs the gastric mill retractor phase, via presynaptic inhibition of MCN1. In the absence of GPR stimulation, CCAP does not alter retraction duration and modestly prolongs protraction. Here, we show, using computational modeling and dynamic-clamp manipulations, that the presence of CCAP weakens or eliminates the GPR effect on the gastric mill rhythm. This CCAP action results from its ability to activate the same modulator-activated conductance (G(MI)) as MCN1 in the gastric mill circuit neuron lateral gastric (LG). Because GPR prolongs retraction by weakening MCN1 activation of G(MI) in LG, the parallel G(MI) activation by CCAP reduces the impact of GPR regulation of this conductance. The CCAP-activated G(MI) thus counteracts the GPR-mediated decrease in the MCN1-activated G(MI) in LG and reduces the GPR ability to regulate the gastric mill rhythm. Consequently, although CCAP neither changes retraction duration nor alters GPR inhibition of MCN1, its activation of a modulator-activated conductance in a pivotal downstream circuit neuron enables CCAP to weaken or eliminate sensory regulation of motor circuit output.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
