Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B
- PMID: 20164332
- PMCID: PMC2836844
- DOI: 10.1523/JNEUROSCI.5225-09.2010
Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B
Abstract
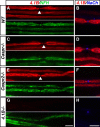
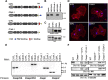
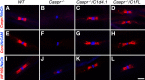
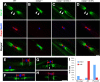
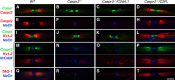
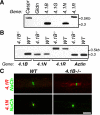
Caspr and Caspr2 regulate the formation of distinct axonal domains around the nodes of Ranvier. Caspr is required for the generation of a membrane barrier at the paranodal junction (PNJ), whereas Caspr2 serves as a membrane scaffold that clusters Kv1 channels at the juxtaparanodal region (JXP). Both Caspr and Caspr2 interact with protein 4.1B, which may link the paranodal and juxtaparanodal adhesion complexes to the axonal cytoskeleton. To determine the role of protein 4.1B in the function of Caspr proteins, we examined the ability of transgenic Caspr and Caspr2 mutants lacking their 4.1-binding sequence (d4.1) to restore Kv1 channel clustering in Caspr- and Caspr2-null mice, respectively. We found that Caspr-d4.1 was localized to the PNJ and is able to recruit the paranodal adhesion complex components contactin and NF155 to this site. Nevertheless, in axons expressing Caspr-d4.1, Kv1 channels were often detected at paranodes, suggesting that the interaction of Caspr with protein 4.1B is necessary for the generation of an efficient membrane barrier at the PNJ. We also found that the Caspr2-d4.1 transgene did not accumulate at the JXP, even though it was targeted to the axon, demonstrating that the interaction with protein 4.1B is required for the accumulation of Caspr2 and Kv1 channels at the juxtaparanodal axonal membrane. In accordance, we show that Caspr2 and Kv1 channels are not clustered at the JXP in 4.1B-null mice. Our results thus underscore the functional importance of protein 4.1B in the organization of peripheral myelinated axons.
Figures

References
-
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol. 1999;28:333–347. - PubMed
-
- Arroyo EJ, Sirkowski EE, Chitale R, Scherer SS. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479:424–434. - PubMed
-
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. - PubMed
-
- Beekman JM, Bakema JE, van der Poel CE, van der Linden JA, van de Winkel JG, Leusen JH. Protein 4.1G binds to a unique motif within the Fc gamma RI cytoplasmic tail. Mol Immunol. 2008;45:2069–2075. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
