Deletion of GRK1 causes retina degeneration through a transducin-independent mechanism
- PMID: 20164334
- PMCID: PMC2849294
- DOI: 10.1523/JNEUROSCI.6254-09.2010
Deletion of GRK1 causes retina degeneration through a transducin-independent mechanism
Abstract
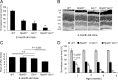
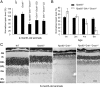
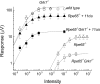
Rpe65(-/-) mice are unable to produce 11-cis-retinal, the chromophore of visual pigments. Consequently, the pigment is present as the apoprotein opsin with a minute level of pigment containing 9-cis-retinal as chromophore. Notably, a 10-20% fraction of this opsin is mono-phosphorylated independently of light conditions. To determine the role of rhodopsin kinase (GRK1) in phosphorylating this opsin and to test whether eliminating this phosphorylation would accelerate photoreceptor degeneration, we generated the Rpe65(-/-)Grk1(-/-) mouse. The retinae of Rpe65(-/-)Grk1(-/-) mice had negligible opsin phosphorylation, extensive degeneration with decreased opsin levels, and diminished light-evoked rod responses relative to Rpe65(-/-) mice. These data show that opsin phosphorylation in the Rpe65(-/-) mouse is due to the action of GRK1 and is neuroprotective. However, despite the higher activity of unphosphorylated opsin, the severe loss of opsin in the rapidly degenerating Rpe65(-/-)Grk1(-/-) mice resulted in lower overall opsin activity and in higher rod sensitivity compared with Rpe65(-/-) mice. In Rpe65(-/-)Grk1(-/-)Gnat1(-/-) mice where transduction activation was blocked, degeneration was only partially prevented. Therefore, increased opsin activity in the absence of phosphorylation was not the only mechanism for the accelerated retinal degeneration. Finally, the deletion of GRK1 triggered retinal degeneration in Grk1(-/-) mice after 1 month, even in the absence of apo-opsin. This degeneration was independent of light conditions and occurred even in the absence of transducin in Grk1(-/-)Gnat1(-/-) mice. Taken together, our results demonstrate a light-independent mechanism for retinal degeneration in the absence of GRK1, suggesting a second, not previously recognized role for that kinase.
Figures



References
-
- Ablonczy Z, Knapp DR, Darrow R, Organisciak DT, Crouch RK. Mass spectrometric analysis of rhodopsin from light damaged rats. Mol Vis. 2000;6:109–115. - PubMed
-
- Ablonczy Z, Crouch RK, Goletz PW, Redmond TM, Knapp DR, Ma JX, Rohrer B. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277:40491–40498. - PubMed
-
- Arshavsky VY. Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci. 2002;25:124–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY006837/EY/NEI NIH HHS/United States
- EY13520/EY/NEI NIH HHS/United States
- R01 EY019312/EY/NEI NIH HHS/United States
- R01 EY014596/EY/NEI NIH HHS/United States
- R56 EY013811/EY/NEI NIH HHS/United States
- EY06837/EY/NEI NIH HHS/United States
- EY02687/EY/NEI NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01 EY004939/EY/NEI NIH HHS/United States
- F32 EY006837/EY/NEI NIH HHS/United States
- R01 DC006904/DC/NIDCD NIH HHS/United States
- EY04939/EY/NEI NIH HHS/United States
- R01 EY013520/EY/NEI NIH HHS/United States
- P30 EY002687/EY/NEI NIH HHS/United States
- R01 EY013811/EY/NEI NIH HHS/United States
- EY19312/EY/NEI NIH HHS/United States
- R24 EY014793/EY/NEI NIH HHS/United States
- EY13811/EY/NEI NIH HHS/United States
- EY14793/EY/NEI NIH HHS/United States
- R37 EY006837/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
