Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice
- PMID: 20164342
- PMCID: PMC2969849
- DOI: 10.1523/JNEUROSCI.4470-09.2010
Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice
Abstract
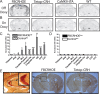
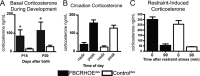
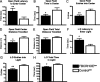
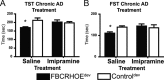
During development, early-life stress, such as abuse or trauma, induces long-lasting changes that are linked to adult anxiety and depressive behavior. It has been postulated that altered expression of corticotropin-releasing hormone (CRH) can at least partially account for the various effects of stress on behavior. In accord with this hypothesis, evidence from pharmacological and genetic studies has indicated the capacity of differing levels of CRH activity in different brain areas to produce behavioral changes. Furthermore, stress during early life or adulthood causes an increase in CRH release in a variety of neural sites. To evaluate the temporal and spatial specificity of the effect of early-life CRH exposure on adult behavior, the tetracycline-off system was used to produce mice with forebrain-restricted inducible expression of CRH. After transient elevation of CRH during development only, behavioral testing in adult mice revealed a persistent anxiogenic and despair-like phenotype. These behavioral changes were not associated with alterations in adult circadian or stress-induced corticosterone release but were associated with changes in CRH receptor type 1 expression. Furthermore, the despair-like changes were normalized with antidepressant treatment. Overall, these studies suggest that forebrain-restricted CRH signaling during development can permanently alter stress adaptation leading to increases in maladaptive behavior in adulthood.
Figures


Similar articles
-
Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1.Eur J Neurosci. 2012 Aug;36(3):2360-7. doi: 10.1111/j.1460-9568.2012.08148.x. Epub 2012 Jun 4. Eur J Neurosci. 2012. PMID: 22672268
-
Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior.Mol Psychiatry. 2008 Nov;13(11):1028-42. doi: 10.1038/mp.2008.51. Epub 2008 May 13. Mol Psychiatry. 2008. PMID: 18475271
-
Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep.Mol Psychiatry. 2010 Feb;15(2):154-65. doi: 10.1038/mp.2009.46. Epub 2009 May 19. Mol Psychiatry. 2010. PMID: 19455148 Free PMC article.
-
Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans.Stress. 2017 Sep;20(5):449-464. doi: 10.1080/10253890.2017.1322575. Epub 2017 May 18. Stress. 2017. PMID: 28436309 Free PMC article. Review.
-
CRH signaling. Molecular specificity for drug targeting in the CNS.Ann N Y Acad Sci. 2009 Oct;1179:106-19. doi: 10.1111/j.1749-6632.2009.04983.x. Ann N Y Acad Sci. 2009. PMID: 19906235 Review.
Cited by
-
Music prevents stress-induced depression and anxiety-like behavior in mice.Transl Psychiatry. 2023 Oct 12;13(1):317. doi: 10.1038/s41398-023-02606-z. Transl Psychiatry. 2023. PMID: 37828015 Free PMC article.
-
Enhancing the Utility of Preclinical Research in Neuropsychiatry Drug Development.Methods Mol Biol. 2019;2011:3-22. doi: 10.1007/978-1-4939-9554-7_1. Methods Mol Biol. 2019. PMID: 31273690 Free PMC article. Review.
-
Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice.Neuropsychopharmacology. 2014 May;39(6):1409-19. doi: 10.1038/npp.2013.336. Epub 2013 Dec 11. Neuropsychopharmacology. 2014. PMID: 24326400 Free PMC article.
-
Central amygdala activation of extracellular signal-regulated kinase 1 and age-dependent changes in inflammatory pain sensitivity in mice.Neurobiol Aging. 2017 Aug;56:100-107. doi: 10.1016/j.neurobiolaging.2017.04.010. Epub 2017 Apr 26. Neurobiol Aging. 2017. PMID: 28526294 Free PMC article.
-
Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning?Nat Rev Drug Discov. 2012 May 18;11(6):462-78. doi: 10.1038/nrd3702. Nat Rev Drug Discov. 2012. PMID: 22596253 Review.
References
-
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–329. - PubMed
-
- Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. - PubMed
-
- Arató M, Bánki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
