Suppression of the intrinsic apoptosis pathway by synaptic activity
- PMID: 20164347
- PMCID: PMC2834927
- DOI: 10.1523/JNEUROSCI.5115-09.2010
Suppression of the intrinsic apoptosis pathway by synaptic activity
Abstract
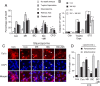
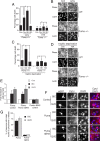
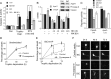
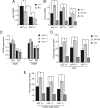
Synaptic activity promotes resistance to diverse apoptotic insults, the mechanism behind which is incompletely understood. We show here that a coordinated downregulation of core components of the intrinsic apoptosis pathway by neuronal activity forms a key part of the underlying mechanism. Activity-dependent protection against apoptotic insults is associated with inhibition of cytochrome c release in most but not all neurons, indicative of anti-apoptotic signaling both upstream and downstream of this step. We find that enhanced firing activity suppresses expression of the proapoptotic BH3-only member gene Puma in a NMDA receptor-dependent, p53-independent manner. Puma expression is sufficient to induce cytochrome c loss and neuronal apoptosis. Puma deficiency protects neurons against apoptosis and also occludes the protective effect of synaptic activity, while blockade of physiological NMDA receptor activity in the developing mouse brain induces neuronal apoptosis that is preceded by upregulation of Puma. However, enhanced activity can also confer resistance to Puma-induced apoptosis, acting downstream of cytochrome c release. This mechanism is mediated by transcriptional suppression of apoptosome components Apaf-1 and procaspase-9, and limiting caspase-9 activity, since overexpression of procaspase-9 accelerates the rate of apoptosis in active neurons back to control levels. Synaptic activity does not exert further significant anti-apoptotic effects downstream of caspase-9 activation, since an inducible form of caspase-9 overrides the protective effect of synaptic activity, despite activity-induced transcriptional suppression of caspase-3. Thus, suppression of apoptotic gene expression may synergize with other activity-dependent events such as enhancement of antioxidant defenses to promote neuronal survival.
Figures


Similar articles
-
The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons.J Neurochem. 2006 Mar;96(5):1213-26. doi: 10.1111/j.1471-4159.2005.03676.x. J Neurochem. 2006. PMID: 16478523
-
BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death.J Neurosci. 2006 Jul 5;26(27):7257-64. doi: 10.1523/JNEUROSCI.0196-06.2006. J Neurosci. 2006. PMID: 16822983 Free PMC article.
-
Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53.J Biol Chem. 2006 Mar 17;281(11):7260-70. doi: 10.1074/jbc.M509868200. Epub 2006 Jan 6. J Biol Chem. 2006. PMID: 16407291
-
Apaf-1: Regulation and function in cell death.Biochimie. 2017 Apr;135:111-125. doi: 10.1016/j.biochi.2017.02.001. Epub 2017 Feb 9. Biochimie. 2017. PMID: 28192157 Review.
-
The influence of synaptic activity on neuronal health.Curr Opin Neurobiol. 2011 Apr;21(2):299-305. doi: 10.1016/j.conb.2011.01.002. Epub 2011 Feb 1. Curr Opin Neurobiol. 2011. PMID: 21292474 Free PMC article. Review.
Cited by
-
Mechanisms of Transsynaptic Degeneration in the Aging Brain.Aging Dis. 2024 Oct 1;15(5):2149-2167. doi: 10.14336/AD.2024.03019. Aging Dis. 2024. PMID: 39191395 Free PMC article. Review.
-
Extrasynaptic NMDA receptor-induced tau overexpression mediates neuronal death through suppressing survival signaling ERK phosphorylation.Cell Death Dis. 2016 Nov 3;7(11):e2449. doi: 10.1038/cddis.2016.329. Cell Death Dis. 2016. PMID: 27809304 Free PMC article.
-
Long-term exercise is a potent trigger for ΔFosB induction in the hippocampus along the dorso-ventral axis.PLoS One. 2013 Nov 25;8(11):e81245. doi: 10.1371/journal.pone.0081245. eCollection 2013. PLoS One. 2013. PMID: 24282574 Free PMC article.
-
GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons.J Neurosci. 2013 Apr 10;33(15):6614-22. doi: 10.1523/JNEUROSCI.0781-13.2013. J Neurosci. 2013. PMID: 23575858 Free PMC article.
-
Apaf1-deficient cortical neurons exhibit defects in axonal outgrowth.Cell Mol Life Sci. 2015 Nov;72(21):4173-91. doi: 10.1007/s00018-015-1927-x. Epub 2015 May 15. Cell Mol Life Sci. 2015. PMID: 25975226 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
