Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density
- PMID: 20164349
- PMCID: PMC2917239
- DOI: 10.1523/JNEUROSCI.1617-09.2010
Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density
Abstract
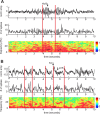
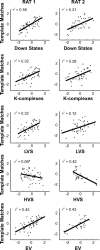
Spontaneous reactivation of previously stored patterns of neural activity occurs in hippocampus and neocortex during non-rapid eye movement (NREM) sleep. Notable features of the neocortical local field potential during NREM sleep are high-amplitude, low-frequency thalamocortical oscillations including K-complexes, low-voltage spindles, and high-voltage spindles. Using combined neuronal ensemble and local field potential recordings, we show that prefrontal stored-trace reactivation is correlated with the density of down-to-up state transitions of the population of simultaneously recorded cells, as well as K-complexes and low-voltage spindles in the local field potential. This result strengthens the connection between reactivation and learning, as these same NREM sleep features have been correlated with memory. Although memory trace reactivation is correlated with low-voltage spindles, it is not correlated with high-voltage spindles, indicating that despite their similar frequency characteristics, these two oscillations serve different functions.
Figures





Similar articles
-
Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep.Neuron. 1998 Nov;21(5):1123-8. doi: 10.1016/s0896-6273(00)80629-7. Neuron. 1998. PMID: 9856467
-
Coupling of Slow Oscillations in the Prefrontal and Motor Cortex Predicts Onset of Spindle Trains and Persistent Memory Reactivations.J Neurosci. 2024 Oct 23;44(43):e0621242024. doi: 10.1523/JNEUROSCI.0621-24.2024. J Neurosci. 2024. PMID: 39168655 Free PMC article.
-
Replay of rule-learning related neural patterns in the prefrontal cortex during sleep.Nat Neurosci. 2009 Jul;12(7):919-26. doi: 10.1038/nn.2337. Epub 2009 May 31. Nat Neurosci. 2009. PMID: 19483687
-
Memory trace reactivation in hippocampal and neocortical neuronal ensembles.Curr Opin Neurobiol. 2000 Apr;10(2):180-6. doi: 10.1016/s0959-4388(00)00079-9. Curr Opin Neurobiol. 2000. PMID: 10753801 Review.
-
A mechanism for learning with sleep spindles.Philos Trans R Soc Lond B Biol Sci. 2020 May 25;375(1799):20190230. doi: 10.1098/rstb.2019.0230. Epub 2020 Apr 6. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32248788 Free PMC article. Review.
Cited by
-
Impaired Hippocampal-Cortical Interactions during Sleep in a Mouse Model of Alzheimer's Disease.Curr Biol. 2020 Jul 6;30(13):2588-2601.e5. doi: 10.1016/j.cub.2020.04.087. Epub 2020 May 28. Curr Biol. 2020. PMID: 32470367 Free PMC article.
-
Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning.Hippocampus. 2015 Oct;25(10):1073-188. doi: 10.1002/hipo.22488. Hippocampus. 2015. PMID: 26135716 Free PMC article. Review.
-
Hippocampal-cortical coupling differentiates long-term memory processes.Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2207909120. doi: 10.1073/pnas.2207909120. Epub 2023 Feb 7. Proc Natl Acad Sci U S A. 2023. PMID: 36749719 Free PMC article.
-
Hippocampal Reactivation Extends for Several Hours Following Novel Experience.J Neurosci. 2019 Jan 30;39(5):866-875. doi: 10.1523/JNEUROSCI.1950-18.2018. Epub 2018 Dec 10. J Neurosci. 2019. PMID: 30530857 Free PMC article.
-
Brain Activation Time-Locked to Sleep Spindles Associated With Human Cognitive Abilities.Front Neurosci. 2019 Feb 6;13:46. doi: 10.3389/fnins.2019.00046. eCollection 2019. Front Neurosci. 2019. PMID: 30787863 Free PMC article.
References
-
- Aldinio C, Aporti F, Calderini G, Mazzari S, Zanotti A, Toffano G. Experimental models of aging and quinolinic acid. Methods Find Exp Clin Pharmacol. 1985;7:563–568. - PubMed
-
- Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. - PubMed
-
- Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. - PubMed
-
- Amzica F, Steriade M. The functional significance of K-complexes. Sleep Med Rev. 2002;6:139–149. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
