Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation
- PMID: 20164351
- PMCID: PMC2840640
- DOI: 10.1523/JNEUROSCI.4022-09.2010
Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation
Abstract
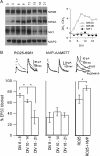
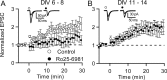
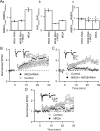
NMDA receptors (NMDARs) are critical mediators of activity-dependent synaptic plasticity, but the differential roles of NR2A- versus NR2B-containing NMDARs have been controversial. Here, we investigate the roles of NR2A and NR2B in long-term potentiation (LTP) in organotypic hippocampal slice cultures using RNA interference (RNAi) and overexpression, to complement pharmacological approaches. In young slices, when NR2B is the predominant subunit expressed, LTP is blocked by the NR2B-selective antagonist Ro25-6981 [R-(R,S)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine propranol]. As slices mature and NR2A expression rises, activation of NR2B receptors became no longer necessary for LTP induction. LTP was blocked, however, by RNAi knockdown of NR2B, and this was rescued by coexpression of an RNAi-resistant NR2B (NR2B*) cDNA. Interestingly, a chimeric NR2B subunit in which the C-terminal cytoplasmic tail was replaced by that of NR2A failed to rescue LTP, whereas the reverse chimera, NR2A channel with NR2B tail, was able to restore LTP. Thus, expression of NR2B with its intact cytoplasmic tail is required for LTP induction, at an age when channel activity of NR2B-NMDARs is not required for LTP. Overexpression of wild-type NR2A failed to rescue LTP in neurons transfected with the NR2B-RNAi construct, despite restoring NMDA-EPSC amplitude to a similar level as NR2B*. Surprisingly, an NR2A construct lacking its entire C-terminal cytoplasmic tail regained its ability to restore LTP. Together, these data suggest that the NR2B subunit plays a critical role for LTP, presumably by recruiting relevant molecules important for LTP via its cytoplasmic tail. In contrast, NR2A is not essential for LTP, and its cytoplasmic tail seems to carry inhibitory factors for LTP.
Figures




Similar articles
-
Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors.Eur J Neurosci. 2008 Apr;27(8):1957-64. doi: 10.1111/j.1460-9568.2008.06173.x. Eur J Neurosci. 2008. PMID: 18412616
-
Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus.Neuropharmacology. 2007 Jan;52(1):60-70. doi: 10.1016/j.neuropharm.2006.07.013. Epub 2006 Aug 10. Neuropharmacology. 2007. PMID: 16904707
-
Late phase of long-term potentiation induced by co-application of N-methyl-d-aspartic acid and the antagonist of NR2B-containing N-methyl-d-aspartic acid receptors in rat hippocampus.Neuroscience. 2009 Mar 3;159(1):127-35. doi: 10.1016/j.neuroscience.2008.10.037. Epub 2008 Oct 30. Neuroscience. 2009. PMID: 19010396
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity.Neuropharmacology. 2008 Dec;55(7):1081-94. doi: 10.1016/j.neuropharm.2008.07.046. Epub 2008 Aug 8. Neuropharmacology. 2008. PMID: 18755202 Free PMC article. Review.
Cited by
-
Hippocampal Memory Recovery After Acute Stress: A Behavioral, Morphological and Molecular Study.Front Mol Neurosci. 2018 Aug 17;11:283. doi: 10.3389/fnmol.2018.00283. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30174589 Free PMC article.
-
Dynamic regulation of NMDA receptor transmission.J Neurophysiol. 2011 Jan;105(1):162-71. doi: 10.1152/jn.00457.2010. Epub 2010 Oct 27. J Neurophysiol. 2011. PMID: 20980539 Free PMC article.
-
Role of GluN2A NMDA receptor in homocysteine-induced prostaglandin E2 release from neurons.J Neurochem. 2019 Jul;150(1):44-55. doi: 10.1111/jnc.14775. Epub 2019 Jun 20. J Neurochem. 2019. PMID: 31125437 Free PMC article.
-
PSD95 suppresses dendritic arbor development in mature hippocampal neurons by occluding the clustering of NR2B-NMDA receptors.PLoS One. 2014 Apr 4;9(4):e94037. doi: 10.1371/journal.pone.0094037. eCollection 2014. PLoS One. 2014. PMID: 24705401 Free PMC article.
-
Metabotropic NMDAR Signaling Contributes to Sex Differences in Synaptic Plasticity and Episodic Memory.J Neurosci. 2024 Dec 11;44(50):e0438242024. doi: 10.1523/JNEUROSCI.0438-24.2024. J Neurosci. 2024. PMID: 39424366 Free PMC article.
References
-
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. - PubMed
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
