Saccade suppression by electrical microstimulation in monkey caudate nucleus
- PMID: 20164354
- PMCID: PMC6634530
- DOI: 10.1523/JNEUROSCI.5011-09.2010
Saccade suppression by electrical microstimulation in monkey caudate nucleus
Abstract
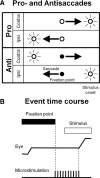
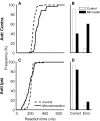
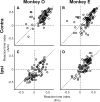
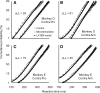
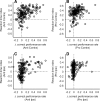
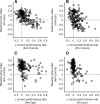
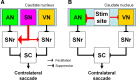
It has been suggested that the caudate nucleus, the input stage of the basal ganglia, facilitates and suppresses saccade initiation based on its anatomical characteristics. Although the involvement of the caudate nucleus in saccade facilitation has been shown previously, it is still unclear whether the caudate nucleus is also involved in saccade suppression. Here, we revealed the direct involvement of the caudate nucleus in saccade suppression by electrical microstimulation in behaving monkeys. We delivered microstimulation to the caudate nucleus while monkeys performed the prosaccade (look toward a peripheral visual stimulus) and antisaccade (look away from the stimulus) paradigm. The reaction times of contralateral saccades were prolonged on both prosaccade and antisaccade trials. The suppression effects on reaction times were stronger on prosaccade trials compared with antisaccade trials. The analysis of reaction time distributions using the linear approach to threshold with ergodic rate model (LATER model) revealed that microstimulation prolonged reaction times by reducing the rate of rise to the threshold for saccade initiation. Microstimulation also worsened correct performance rates for contralateral saccades. The same microstimulation prolonged and/or shortened the reaction times of ipsilateral saccades, although the effects were not as consistent as those on contralateral saccades. We conclude that caudate signals are sufficient to suppress contralateral saccades and influence saccadic decision by controlling contralateral and ipsilateral saccade commands at the same time.
Figures


Similar articles
-
Saccade reaction times are influenced by caudate microstimulation following and prior to visual stimulus appearance.J Cogn Neurosci. 2011 Jul;23(7):1794-807. doi: 10.1162/jocn.2010.21554. Epub 2010 Jul 28. J Cogn Neurosci. 2011. PMID: 20666599
-
Effects of caudate microstimulation on spontaneous and purposive saccades.J Neurophysiol. 2013 Jul;110(2):334-43. doi: 10.1152/jn.00046.2013. Epub 2013 May 1. J Neurophysiol. 2013. PMID: 23636720
-
Effects of anterior cingulate microstimulation on pro- and antisaccades in nonhuman primates.J Cogn Neurosci. 2011 Feb;23(2):481-90. doi: 10.1162/jocn.2010.21482. Epub 2010 Mar 29. J Cogn Neurosci. 2011. PMID: 20350174
-
Saccade dysmetria during functional perturbation of the caudal fastigial nucleus in the monkey.Ann N Y Acad Sci. 2003 Oct;1004:220-8. doi: 10.1196/annals.1303.019. Ann N Y Acad Sci. 2003. PMID: 14662461 Review.
-
Direct and indirect pathways for choosing objects and actions.Eur J Neurosci. 2019 Mar;49(5):637-645. doi: 10.1111/ejn.13876. Epub 2018 Mar 25. Eur J Neurosci. 2019. PMID: 29473660 Free PMC article. Review.
Cited by
-
Cognitive control of gaze in bipolar disorder and schizophrenia.Psychiatry Res. 2015 Feb 28;225(3):254-62. doi: 10.1016/j.psychres.2014.12.033. Epub 2014 Dec 31. Psychiatry Res. 2015. PMID: 25601802 Free PMC article.
-
Dissociable effects of dopamine on learning and performance within sensorimotor striatum.Basal Ganglia. 2014 Jun 1;4(2):43-54. doi: 10.1016/j.baga.2013.11.001. Basal Ganglia. 2014. PMID: 24949283 Free PMC article.
-
Control of response interference: caudate nucleus contributes to selective inhibition.Sci Rep. 2020 Dec 1;10(1):20977. doi: 10.1038/s41598-020-77744-1. Sci Rep. 2020. PMID: 33262369 Free PMC article.
-
A novel, variable angle guide grid for neuronal activity studies.Front Integr Neurosci. 2012 Jan 20;6:1. doi: 10.3389/fnint.2012.00001. eCollection 2011 Dec 4. Front Integr Neurosci. 2012. PMID: 22319479 Free PMC article.
-
Proactive selective response suppression is implemented via the basal ganglia.J Neurosci. 2013 Aug 14;33(33):13259-69. doi: 10.1523/JNEUROSCI.5651-12.2013. J Neurosci. 2013. PMID: 23946385 Free PMC article.
References
-
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:191–204.
-
- Bell AH, Everling S, Munoz DP. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J Neurophysiol. 2000;84:2595–2604. - PubMed
-
- Briand KA, Strallow D, Hening W, Poizner H, Sereno AB. Control of voluntary and reflexive saccades in parkinson's disease. Exp Brain Res. 1999;129:38–48. - PubMed
-
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. - PubMed
-
- Cebrián C, Parent A, Prensa L. Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J Comp Neurol. 2005;492:349–369. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
