Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity
- PMID: 20164356
- PMCID: PMC3842473
- DOI: 10.1523/JNEUROSCI.3903-09.2010
Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity
Abstract
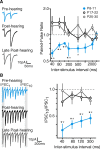
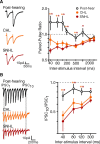
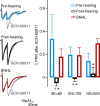
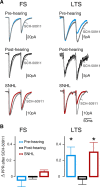
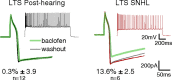
Short-term changes in synaptic gain support information processing throughout the CNS, yet we know little about the developmental regulation of such plasticity. Here we report that auditory experience is necessary for the normal maturation of synaptic inhibitory short-term plasticity (iSTP) in the auditory cortex, and that presynaptic GABA(B) receptors regulate this development. Moderate or severe hearing loss was induced in gerbils, and iSTP was characterized by measuring inhibitory synaptic current amplitudes in response to repetitive stimuli. We reveal a profound developmental shift of iSTP from depressing to facilitating after the onset of hearing. Even moderate hearing loss prevented this shift. This iSTP change was mediated by a specific class of inhibitory interneurons, the low-threshold spiking cells. Further, using paired recordings, we reveal that presynaptic GABA(B) receptors at interneuron-pyramidal connections regulate iSTP in an experience-dependent manner. This novel synaptic mechanism may support the emergence of mature temporal processing in the auditory cortex.
Figures



Similar articles
-
Pre- and postsynaptic inhibition mediated by GABA(B) receptors in cerebellar inhibitory interneurons.J Neurophysiol. 2002 Jan;87(1):183-90. doi: 10.1152/jn.00344.2001. J Neurophysiol. 2002. PMID: 11784741
-
Cell-type specific GABA synaptic transmission and activity-dependent plasticity in rat hippocampal stratum radiatum interneurons.Eur J Neurosci. 2005 Jul;22(1):179-88. doi: 10.1111/j.1460-9568.2005.04207.x. Eur J Neurosci. 2005. PMID: 16029207
-
Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype.J Neurosci. 2012 Jan 18;32(3):983-8. doi: 10.1523/JNEUROSCI.5007-11.2012. J Neurosci. 2012. PMID: 22262896 Free PMC article.
-
Developmental plasticity of auditory cortical inhibitory synapses.Hear Res. 2011 Sep;279(1-2):140-8. doi: 10.1016/j.heares.2011.03.015. Epub 2011 Apr 2. Hear Res. 2011. PMID: 21463668 Free PMC article. Review.
-
A behavioral framework to guide research on central auditory development and plasticity.Neuron. 2011 Dec 22;72(6):912-29. doi: 10.1016/j.neuron.2011.12.005. Neuron. 2011. PMID: 22196328 Free PMC article. Review.
Cited by
-
Biological impact of auditory expertise across the life span: musicians as a model of auditory learning.Hear Res. 2014 Feb;308:109-21. doi: 10.1016/j.heares.2013.08.004. Epub 2013 Aug 26. Hear Res. 2014. PMID: 23988583 Free PMC article. Review.
-
Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies.J Assoc Res Otolaryngol. 2011 Oct;12(5):535-47. doi: 10.1007/s10162-011-0271-6. Epub 2011 May 24. J Assoc Res Otolaryngol. 2011. PMID: 21607783 Free PMC article. Review.
-
Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex.Sci Rep. 2017 Dec 12;7(1):17423. doi: 10.1038/s41598-017-17711-5. Sci Rep. 2017. PMID: 29234064 Free PMC article.
-
Developmental shift of short-term synaptic plasticity in cortical organotypic slices.Neuroscience. 2012 Jun 28;213:38-46. doi: 10.1016/j.neuroscience.2012.04.018. Epub 2012 Apr 19. Neuroscience. 2012. PMID: 22521823 Free PMC article.
-
GABAergic synapses: their plasticity and role in sensory cortex.Front Cell Neurosci. 2014 Mar 26;8:91. doi: 10.3389/fncel.2014.00091. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24723851 Free PMC article. Review.
References
-
- Aizawa N, Eggermont JJ. Mild noise-induced hearing loss affects temporal modulation transfer functions in adult cat primary auditory cortex. Hear Res. 2007;223:71–82. - PubMed
-
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. Eur J Pharmacol. 1991;199:259–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
