Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors
- PMID: 20164358
- PMCID: PMC2862277
- DOI: 10.1523/JNEUROSCI.5390-09.2010
Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors
Abstract
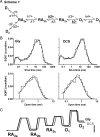
We have studied relative efficacies of NR1 agonists glycine and d-cycloserine (DCS), and found efficacy to be dependent on the NR2 subunit. DCS shows partial agonism at NR1/NR2B but has higher relative efficacy than glycine at NR1/NR2C receptor. Molecular dynamics (MD) simulations of the NR1/NR2B and NR1/NR2C agonist binding domain dimer suggest only subtle differences in the interactions of DCS with NR1 binding site residues relative to glycine. The most pronounced differences were observed in the NR1/NR2C simulation between the orientation of helices F and G of the NR1 subunit. Interestingly, Helix F was previously proposed to influence receptor gating and to adopt an orientation depending on agonist efficacy. MD simulations and site-directed mutagenesis further suggest a role for residues at the agonist binding domain dimer interface in regulating DCS efficacy. To relate the structural rearrangements to receptor gating, we recorded single-channel currents from outside-out patches containing a single active NR1/NR2C receptor. DCS increased the mean open time and open probability of NR1/NR2C receptors compared with glycine. Maximum likelihood fitting of a gating model for NR1/NR2C receptor activation to the single-channel data suggests that DCS specifically accelerates the rate constant governing a fast gating step and reduces the closing rate. These changes appear to reflect a decreased activation energy for a pregating step and increased stability of the open states. We suggest that the higher efficacy of DCS at NR1/NR2C receptors involves structural rearrangements at the dimer interface and an effect on NR1/NR2C receptor pregating conformational changes.
Figures





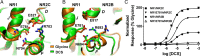


Similar articles
-
Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine.Neuropharmacology. 2001 Aug;41(2):151-8. doi: 10.1016/s0028-3908(01)00073-9. Neuropharmacology. 2001. PMID: 11489451
-
Activation of recombinant NR1/NR2C NMDA receptors.J Physiol. 2008 Sep 15;586(18):4425-39. doi: 10.1113/jphysiol.2008.158634. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635641 Free PMC article.
-
Subunit-specific contribution of pore-forming domains to NMDA receptor channel structure and gating.J Gen Physiol. 2007 Jun;129(6):509-25. doi: 10.1085/jgp.200609718. Epub 2007 May 15. J Gen Physiol. 2007. PMID: 17504910 Free PMC article.
-
N-[2-(N-(2-mercaptoethyl)) amino ethyl]-N-(2-mercaptoethyl)-3,5-dimethylacetamide amantadine-technetium.2012 Jun 12 [updated 2012 Jul 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jun 12 [updated 2012 Jul 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22812023 Free Books & Documents. Review.
-
Potential of D-cycloserine in the treatment of behavioral and neuroinflammatory disorders in Parkinson's disease and studies that need to be performed before clinical trials.Kaohsiung J Med Sci. 2012 Aug;28(8):407-17. doi: 10.1016/j.kjms.2012.02.010. Epub 2012 Apr 22. Kaohsiung J Med Sci. 2012. PMID: 22892161 Free PMC article. Review.
Cited by
-
d-Cycloserine augmentation of cognitive remediation in schizophrenia.Schizophr Res. 2014 Mar;153(1-3):177-83. doi: 10.1016/j.schres.2014.01.016. Epub 2014 Jan 30. Schizophr Res. 2014. PMID: 24485587 Free PMC article. Clinical Trial.
-
D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia.Schizophr Res. 2011 Sep;131(1-3):69-74. doi: 10.1016/j.schres.2011.05.029. Epub 2011 Jun 30. Schizophr Res. 2011. PMID: 21723096 Free PMC article. Clinical Trial.
-
Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator.Mol Pharmacol. 2014 Nov;86(5):548-60. doi: 10.1124/mol.114.094516. Epub 2014 Sep 9. Mol Pharmacol. 2014. PMID: 25205677 Free PMC article.
-
Functional insights from glutamate receptor ion channel structures.Annu Rev Physiol. 2013;75:313-37. doi: 10.1146/annurev-physiol-030212-183711. Epub 2012 Sep 4. Annu Rev Physiol. 2013. PMID: 22974439 Free PMC article. Review.
-
Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond.Biol Psychiatry. 2013 Jun 15;73(12):1133-41. doi: 10.1016/j.biopsych.2013.03.026. Biol Psychiatry. 2013. PMID: 23726151 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
