Coordination between extrinsic extracellular matrix cues and intrinsic responses to orient the centrosome in polarizing cerebellar granule neurons
- PMID: 20164359
- PMCID: PMC2846173
- DOI: 10.1523/JNEUROSCI.4218-09.2010
Coordination between extrinsic extracellular matrix cues and intrinsic responses to orient the centrosome in polarizing cerebellar granule neurons
Abstract
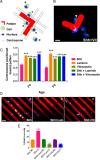
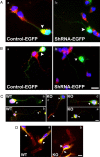
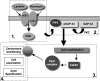
Successful axon targeting during development is critically dependent on directionality of axon extension and requires coordination between the extrinsic cues that provide spatial information to the axon and the intrinsic responses that regulate structural specification of the axon during neuronal polarization. How these responses are coordinated is unclear but are known to involve aligning the centrosome with the base of the emerging axon. We have used a novel in vitro micropatterning assay that spatially segregates the extrinsic cues used by polarizing cerebellar granule cells to orient axon extension and used it to investigate the signaling mechanisms responsible for coordinating centrosome positioning with intrinsic responses. The results show that, when laminin and/or vitronectin are used as spatially restricted cues in association with substrate-associated sonic hedgehog, they are sufficient to induce cell cycle arrest, that laminin and vitronectin then induce integrin-mediated signaling that upregulates phosphoinositide-3 kinase and PKC function to produce phosphatidylinositol 3,4,5-trisphosphate (PIP3) that is associated with the centrosome, that this PIP3 can interact with PKC-phosphorylated growth-associated protein GAP-43, and that PKC-phosphorylated GAP-43 in turn is required for positioning Par6, Cdc42, and IQGAP1, all intrinsic response components, in proximity to the centrosome, such that, in the absence of GAP-43, they are mislocalized and microtubules are not oriented appropriately. We conclude from these results that GAP-43 plays an important role in coordinating extrinsic signaling and intrinsic responses in polarizing cerebellar granule neurons.
Figures







Similar articles
-
GAP-43 is key to mitotic spindle control and centrosome-based polarization in neurons.Cell Cycle. 2008 Feb 1;7(3):348-57. doi: 10.4161/cc.7.3.5235. Epub 2007 Nov 1. Cell Cycle. 2008. PMID: 18235238
-
Beta1-integrins are critical for cerebellar granule cell precursor proliferation.J Neurosci. 2004 Mar 31;24(13):3402-12. doi: 10.1523/JNEUROSCI.5241-03.2004. J Neurosci. 2004. PMID: 15056720 Free PMC article.
-
14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing.Neuron. 2012 Nov 21;76(4):735-49. doi: 10.1016/j.neuron.2012.09.017. Neuron. 2012. PMID: 23177959
-
The extracellular matrix provides directional cues for neuronal migration during cerebellar development.Braz J Med Biol Res. 2006 Mar;39(3):313-20. doi: 10.1590/s0100-879x2006000300001. Epub 2006 Feb 22. Braz J Med Biol Res. 2006. PMID: 16501810 Review.
-
PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization.Cell Mol Life Sci. 2018 Aug;75(15):2735-2761. doi: 10.1007/s00018-018-2828-6. Epub 2018 Apr 25. Cell Mol Life Sci. 2018. PMID: 29696344 Free PMC article. Review.
Cited by
-
Cell polarity: having and making sense of direction-on the evolutionary significance of the primary cilium/centrosome organ in Metazoa.Open Biol. 2018 Aug;8(8):180052. doi: 10.1098/rsob.180052. Open Biol. 2018. PMID: 30068565 Free PMC article. Review.
-
Centrosome movements in vivo correlate with specific neurite formation downstream of LIM homeodomain transcription factor activity.Development. 2012 Oct;139(19):3590-9. doi: 10.1242/dev.081513. Epub 2012 Aug 16. Development. 2012. PMID: 22899847 Free PMC article.
-
Microtubules Modulate F-actin Dynamics during Neuronal Polarization.Sci Rep. 2017 Aug 29;7(1):9583. doi: 10.1038/s41598-017-09832-8. Sci Rep. 2017. PMID: 28851982 Free PMC article.
-
Vitronectin is Involved in the Morphological Transition of Neurites in Retinoic Acid-Induced Neurogenesis of Neuroblastoma Cell Line Neuro2a.Neurochem Res. 2019 Jul;44(7):1621-1635. doi: 10.1007/s11064-019-02787-4. Epub 2019 Apr 1. Neurochem Res. 2019. PMID: 30937689
-
Heterocellular Contacts with Mouse Brain Endothelial Cells Via Laminin and α6β1 Integrin Sustain Subventricular Zone (SVZ) Stem/Progenitor Cells Properties.Front Cell Neurosci. 2016 Dec 15;10:284. doi: 10.3389/fncel.2016.00284. eCollection 2016. Front Cell Neurosci. 2016. PMID: 28018177 Free PMC article.
References
-
- Calderon de Anda F, Gärtner A, Tsai LH, Dotti CG. Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci. 2008;121:178–185. - PubMed
-
- Changelian PS, Meiri K, Soppet D, Valenza H, Loewy A, Willard M. Purification of the growth-associated protein GAP-43 by reversed phase chromatography: amino acid sequence analysis and cDNA identification. Brain Res. 1990;510:259–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
