Phase response curve analysis of a full morphological globus pallidus neuron model reveals distinct perisomatic and dendritic modes of synaptic integration
- PMID: 20164360
- PMCID: PMC2833015
- DOI: 10.1523/JNEUROSCI.3959-09.2010
Phase response curve analysis of a full morphological globus pallidus neuron model reveals distinct perisomatic and dendritic modes of synaptic integration
Abstract
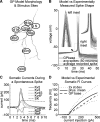
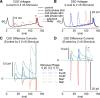
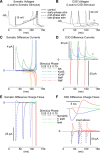
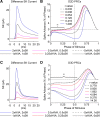
Synchronization of globus pallidus (GP) neurons and cortically entrained oscillations between GP and other basal ganglia nuclei are key features of the pathophysiology of Parkinson's disease. Phase response curves (PRCs), which tabulate the effects of phasic inputs within a neuron's spike cycle on output spike timing, are efficient tools for predicting the emergence of synchronization in neuronal networks and entrainment to periodic input. In this study we apply physiologically realistic synaptic conductance inputs to a full morphological GP neuron model to determine the phase response properties of the soma and different regions of the dendritic tree. We find that perisomatic excitatory inputs delivered throughout the interspike interval advance the phase of the spontaneous spike cycle yielding a type I PRC. In contrast, we demonstrate that distal dendritic excitatory inputs can either delay or advance the next spike depending on whether they occur early or late in the spike cycle. We find this latter pattern of responses, summarized by a biphasic (type II) PRC, was a consequence of dendritic activation of the small conductance calcium-activated potassium current, SK. We also evaluate the spike-frequency dependence of somatic and dendritic PRC shapes, and we demonstrate the robustness of our results to variations of conductance densities, distributions, and kinetic parameters. We conclude that the distal dendrite of GP neurons embodies a distinct dynamical subsystem that could promote synchronization of pallidal networks to excitatory inputs. These results highlight the need to consider different effects of perisomatic and dendritic inputs in the control of network behavior.
Figures




Similar articles
-
Robustness, variability, phase dependence, and longevity of individual synaptic input effects on spike timing during fluctuating synaptic backgrounds: a modeling study of globus pallidus neuron phase response properties.Neuroscience. 2012 Sep 6;219:92-110. doi: 10.1016/j.neuroscience.2012.05.059. Epub 2012 Jun 1. Neuroscience. 2012. PMID: 22659567 Free PMC article.
-
Sodium channels and dendritic spike initiation at excitatory synapses in globus pallidus neurons.J Neurosci. 2004 Jan 14;24(2):329-40. doi: 10.1523/JNEUROSCI.3937-03.2004. J Neurosci. 2004. PMID: 14724231 Free PMC article.
-
The capabilities and limitations of conductance-based compartmental neuron models with reduced branched or unbranched morphologies and active dendrites.J Comput Neurosci. 2011 Apr;30(2):301-21. doi: 10.1007/s10827-010-0258-z. Epub 2010 Jul 10. J Comput Neurosci. 2011. PMID: 20623167 Free PMC article.
-
Functional connectivity and integrative properties of globus pallidus neurons.Neuroscience. 2011 Dec 15;198:44-53. doi: 10.1016/j.neuroscience.2011.07.050. Epub 2011 Jul 27. Neuroscience. 2011. PMID: 21835227 Free PMC article. Review.
-
Dendritic transformations on random synaptic inputs as measured from a neuron's spike train--modeling and simulation.IEEE Trans Biomed Eng. 1989 Jan;36(1):44-54. doi: 10.1109/10.16448. IEEE Trans Biomed Eng. 1989. PMID: 2646212 Review.
Cited by
-
Responses of a bursting pacemaker to excitation reveal spatial segregation between bursting and spiking mechanisms.J Comput Neurosci. 2011 Oct;31(2):419-40. doi: 10.1007/s10827-011-0319-y. Epub 2011 Mar 1. J Comput Neurosci. 2011. PMID: 21360137 Free PMC article.
-
Robustness, variability, phase dependence, and longevity of individual synaptic input effects on spike timing during fluctuating synaptic backgrounds: a modeling study of globus pallidus neuron phase response properties.Neuroscience. 2012 Sep 6;219:92-110. doi: 10.1016/j.neuroscience.2012.05.059. Epub 2012 Jun 1. Neuroscience. 2012. PMID: 22659567 Free PMC article.
-
Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways.J Neurosci. 2014 Feb 5;34(6):2087-99. doi: 10.1523/JNEUROSCI.4646-13.2014. J Neurosci. 2014. PMID: 24501350 Free PMC article.
-
Differential effects of conductances on the phase resetting curve of a bursting neuronal oscillator.J Comput Neurosci. 2015 Jun;38(3):539-58. doi: 10.1007/s10827-015-0553-9. Epub 2015 Apr 3. J Comput Neurosci. 2015. PMID: 25835323 Free PMC article.
-
Modulation of motoneuron firing by recurrent inhibition in the adult rat in vivo.J Neurophysiol. 2014 Nov 1;112(9):2302-15. doi: 10.1152/jn.00358.2014. Epub 2014 Aug 13. J Neurophysiol. 2014. PMID: 25122713 Free PMC article.
References
-
- Abouzeid A, Ermentrout B. Type-II phase resetting curve is optimal for stochastic synchrony. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80 011911. - PubMed
-
- Acker CD, Kopell N, White JA. Synchronization of strongly coupled excitatory neurons: relating network behavior to biophysics. J Comput Neurosci. 2003;15:71–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
