Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors
- PMID: 20164362
- PMCID: PMC2837927
- DOI: 10.1523/JNEUROSCI.3780-09.2010
Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors
Abstract
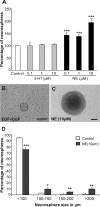
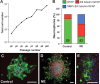
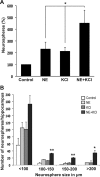
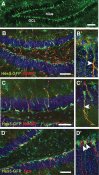
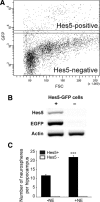
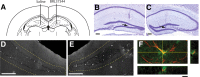
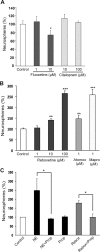
Adult hippocampal neurogenesis is a critical form of cellular plasticity that is greatly influenced by neural activity. Among the neurotransmitters that are widely implicated in regulating this process are serotonin and norepinephrine, levels of which are modulated by stress, depression and clinical antidepressants. However, studies to date have failed to address a direct role for either neurotransmitter in regulating hippocampal precursor activity. Here we show that norepinephrine but not serotonin directly activates self-renewing and multipotent neural precursors, including stem cells, from the hippocampus of adult mice. Mechanistically, we provide evidence that beta(3)-adrenergic receptors, which are preferentially expressed on a Hes5-expressing precursor population in the subgranular zone (SGZ), mediate this norepinephrine-dependent activation. Moreover, intrahippocampal injection of a selective beta(3)-adrenergic receptor agonist in vivo increases the number of proliferating cells in the SGZ. Similarly, systemic injection of the beta-adrenergic receptor agonist isoproterenol not only results in enhancement of proliferation in the SGZ but also leads to an increase in the percentage of nestin/glial fibrillary acidic protein double-positive neural precursors in vivo. Finally, using a novel ex vivo "slice-sphere" assay that maintains an intact neurogenic niche, we demonstrate that antidepressants that selectively block the reuptake of norepinephrine, but not serotonin, robustly increase hippocampal precursor activity via beta-adrenergic receptors. These findings suggest that the activation of neurogenic precursors and stem cells via beta(3)-adrenergic receptors could be a potent mechanism to increase neuronal production, providing a putative target for the development of novel antidepressants.
Figures


Similar articles
-
Opposing effects of α2- and β-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis.PLoS One. 2014 Jun 12;9(6):e98736. doi: 10.1371/journal.pone.0098736. eCollection 2014. PLoS One. 2014. PMID: 24922313 Free PMC article.
-
Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment.J Neurosci. 2010 Jan 20;30(3):1096-109. doi: 10.1523/JNEUROSCI.2309-09.2010. J Neurosci. 2010. PMID: 20089918 Free PMC article.
-
Noggin expands neural stem cells in the adult hippocampus.J Neurosci. 2008 Sep 10;28(37):9194-204. doi: 10.1523/JNEUROSCI.3314-07.2008. J Neurosci. 2008. PMID: 18784300 Free PMC article.
-
The beta3-adrenoceptor as a therapeutic target: current perspectives.Pharmacol Res. 2009 Apr;59(4):221-34. doi: 10.1016/j.phrs.2009.01.002. Epub 2009 Jan 24. Pharmacol Res. 2009. PMID: 19429463 Review.
-
Everything You Always Wanted to Know about β3-AR * (* But Were Afraid to Ask).Cells. 2019 Apr 16;8(4):357. doi: 10.3390/cells8040357. Cells. 2019. PMID: 30995798 Free PMC article. Review.
Cited by
-
Stimulation of the muscarinic receptor M4 regulates neural precursor cell proliferation and promotes adult hippocampal neurogenesis.Development. 2024 Jan 1;151(1):dev201835. doi: 10.1242/dev.201835. Epub 2024 Jan 2. Development. 2024. PMID: 38063486 Free PMC article.
-
Noradrenergic Hypothesis Linking Neurodegeneration-Based Cognitive Decline and Astroglia.Front Mol Neurosci. 2018 Jul 27;11:254. doi: 10.3389/fnmol.2018.00254. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30100866 Free PMC article. Review.
-
The parasympathetic nervous system in the quest for stroke therapeutics.J Cereb Blood Flow Metab. 2011 May;31(5):1187-95. doi: 10.1038/jcbfm.2011.24. Epub 2011 Mar 2. J Cereb Blood Flow Metab. 2011. PMID: 21364605 Free PMC article. Review.
-
Opposing effects of α2- and β-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis.PLoS One. 2014 Jun 12;9(6):e98736. doi: 10.1371/journal.pone.0098736. eCollection 2014. PLoS One. 2014. PMID: 24922313 Free PMC article.
-
Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior.Hum Mol Genet. 2015 Oct 15;24(20):5805-27. doi: 10.1093/hmg/ddv301. Epub 2015 Jul 28. Hum Mol Genet. 2015. PMID: 26220976 Free PMC article.
References
-
- Basak O, Taylor V. Identification of self-replicating multipotent progenitors in the embryonic nervous system by high Notch activity and Hes5 expression. Eur J Neurosci. 2007;25:1006–1022. - PubMed
-
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. - PubMed
-
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. - PubMed
-
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl 14):11–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
