The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function
- PMID: 20164375
- PMCID: PMC2867396
- DOI: 10.1152/ajpcell.00454.2009
The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function
Abstract
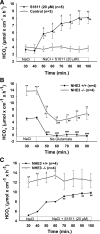
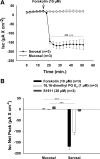
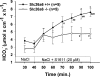
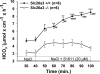
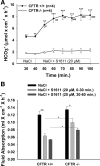
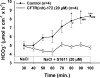
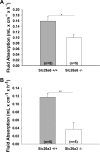
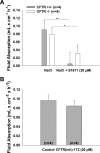
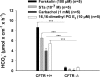
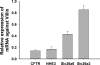
CFTR has been recognized to function as both an anion channel and a key regulator of Slc26 anion transporters in heterologous expression systems. Whether this regulatory relationship between CFTR and Slc26 transporters is seen in native intestine, and whether this effect is coupled to CFTR transport function or other features of this protein, has not been studied. The duodena of anesthetized CFTR-, NHE3-, Slc26a6-, and Scl26a3-deficient mice and wild-type (WT) littermates were perfused, and duodenal bicarbonate (HCO(3)(-)) secretion (DBS) and fluid absorptive or secretory rates were measured. The selective NHE3 inhibitor S1611 or genetic ablation of NHE3 significantly reduced fluid absorptive rates and increased DBS. Slc26a6 (PAT1) or Slc26a3 (DRA) ablation reduced the S1611-induced DBS increase and reduced fluid absorptive rates, suggesting that the effect of S1611 or NHE3 ablation on HCO(3)(-) secretion may be an unmasking of Slc26a6- and Slc26a3-mediated Cl(-)/HCO(3)(-) exchange activity. In the absence of CFTR expression or after application of the CFTR(inh)-172, fluid absorptive rates were similar to those of WT, but S1611 induced virtually no increase in DBS, demonstrating that CFTR transport activity, and not just its presence, is required for Slc26-mediated duodenal HCO(3)(-) secretion. A functionally active CFTR is an absolute requirement for Slc26-mediated duodenal HCO(3)(-) secretion, but not for Slc26-mediated fluid absorption, in which these transporters operate in conjunction with the Na(+)/H(+) exchanger NHE3. This suggests that Slc26a6 and Slc26a3 need proton recycling via NHE3 to operate in the Cl(-) absorptive mode and Cl(-) exit via CFTR to operate in the HCO(3)(-) secretory mode.
Figures
References
-
- Alper SL, Stewart AK, Chernova MN, Zolotarev AS, Clark JS, Vandorpe DH. Anion exchangers in flux: functional differences between human and mouse SLC26A6 polypeptides. Novartis Found Symp 273: 107–119, 2006 - PubMed
-
- Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 - PubMed
-
- Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lunnemann M, Rottinghaus I, Krabbenhoft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, Seidler U. Defective jejunal and colonic salt absorption and altered Na+/H+ exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflügers Arch 457: 1079–1091, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

