TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress
- PMID: 20164382
- PMCID: PMC2889642
- DOI: 10.1152/ajpcell.00458.2009
TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress
Abstract
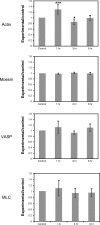
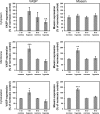
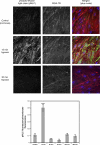
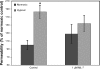
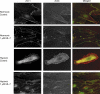
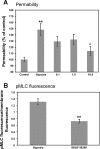
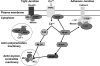
Hypoxia-induced disruption of the blood-brain barrier (BBB) is the result of many different mechanisms, including alterations to the cytoskeleton. In this study, we identified actin-binding proteins involved in cytoskeletal dynamics with quantitative proteomics and assessed changes in subcellular localization of two proteins involved in actin polymerization [vasodilator-stimulated phosphoprotein (VASP)] and cytoskeleton-plasma membrane cross-linking (moesin). We found significant redistribution of both VASP and moesin to the cytoskeletal and membrane fractions of BBB endothelial cells after 1-h hypoxic stress. We also investigated activation of actin-myosin contraction through assessment of phosphorylated myosin light chain (pMLC) with confocal microscopy. Hypoxia caused a rapid and transient increase in pMLC. Blocking MLC phosphorylation through inhibition of myosin light chain kinase (MLCK) with ML-7 prevented hypoxia-induced BBB disruption and relocalization of the tight junction protein ZO-1. Finally, we implicate the transient receptor potential (TRP)C family of channels in mediating these events since blockade of TRPC channels and the associated calcium influx with SKF-96365 prevents hypoxia-induced permeability changes and the phosphorylation of MLC needed for actin-myosin contraction. These data suggest that hypoxic stress triggers alterations to cytoskeletal structure that contribute to BBB disruption and that calcium influx through TRPC channels contributes to these events.
Figures
Similar articles
-
Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement.Microsc Res Tech. 2000 Oct 15;51(2):156-68. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. Microsc Res Tech. 2000. PMID: 11054866
-
Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells.Invest Ophthalmol Vis Sci. 2006 Sep;47(9):4011-8. doi: 10.1167/iovs.05-1127. Invest Ophthalmol Vis Sci. 2006. PMID: 16936117
-
Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption.J Neurochem. 2007 Jul;102(2):501-7. doi: 10.1111/j.1471-4159.2007.04506.x. Epub 2007 Apr 10. J Neurochem. 2007. PMID: 17419808
-
Endothelial calcium dynamics, connexin channels and blood-brain barrier function.Prog Neurobiol. 2013 Sep;108:1-20. doi: 10.1016/j.pneurobio.2013.06.001. Epub 2013 Jul 10. Prog Neurobiol. 2013. PMID: 23851106 Review.
-
Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond.Cells. 2023 Jun 28;12(13):1740. doi: 10.3390/cells12131740. Cells. 2023. PMID: 37443774 Free PMC article. Review.
Cited by
-
Acquiring Metastatic Competence by Oral Squamous Cell Carcinoma Cells Is Associated with Differential Expression of α-Tubulin Isoforms.J Oncol. 2012;2012:491685. doi: 10.1155/2012/491685. Epub 2012 Jun 7. J Oncol. 2012. PMID: 22719762 Free PMC article.
-
Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity.Antioxidants (Basel). 2020 Sep 9;9(9):843. doi: 10.3390/antiox9090843. Antioxidants (Basel). 2020. PMID: 32916895 Free PMC article.
-
Viral disruption of the blood-brain barrier.Trends Microbiol. 2012 Jun;20(6):282-90. doi: 10.1016/j.tim.2012.03.009. Epub 2012 May 6. Trends Microbiol. 2012. PMID: 22564250 Free PMC article. Review.
-
Hypoxia-induced blood-brain barrier dysfunction is prevented by pericyte-conditioned media via attenuated actomyosin contractility and claudin-5 stabilization.FASEB J. 2022 May;36(5):e22331. doi: 10.1096/fj.202200010RR. FASEB J. 2022. PMID: 35476363 Free PMC article.
-
Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement.Cell Signal. 2011 Dec;23(12):2086-96. doi: 10.1016/j.cellsig.2011.08.003. Epub 2011 Aug 12. Cell Signal. 2011. PMID: 21864676 Free PMC article.
References
-
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16: 1–13, 2004 - PubMed
-
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun 327: 1114–1123, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

