Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task
- PMID: 20164392
- PMCID: PMC2867565
- DOI: 10.1152/jn.01075.2009
Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task
Abstract
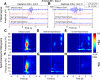
The hippocampus and olfactory regions are anatomically close, and both play a major role in memory formation. However, the way they interact during odor processing is still unclear. In both areas, strong oscillations of the local field potential (LFP) can be recorded, and are modulated by behavior. In particular, in the olfactory system, the beta rhythm (15-35 Hz) is associated with cognitive processing of an olfactory stimulus. Using LFP recordings in the olfactory bulb and dorsal and ventral hippocampus during performance of an olfactory go/no-go task in rats, we previously showed that beta oscillations are also present in the hippocampus, coherent with those in the olfactory bulb, during odor sampling. In this study, we provide further insight into information transfer in the olfacto-hippocampal network by using directional coherence (DCOH estimate), a method based on the temporal relation between two or more signals in the frequency domain. In the theta band (6-12 Hz), coherence between the olfactory bulb (OB) and the hippocampus (HPC) is weak and can be both in the feedback and feedforward directions. However, at this frequency, modulation of the coupling between the dorsal and ventral hippocampus is seen during stimulus expectation versus odor processing. In the beta frequency band (15-35 Hz), analysis showed a strong unidirectional coupling from the OB to dorsal and ventral HPC, indicating that, during odor processing, beta oscillations in the hippocampus are driven by the olfactory bulb.
Figures




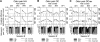

Similar articles
-
An olfacto-hippocampal network is dynamically involved in odor-discrimination learning.J Neurophysiol. 2007 Oct;98(4):2196-205. doi: 10.1152/jn.00524.2007. Epub 2007 Aug 15. J Neurophysiol. 2007. PMID: 17699692
-
Theta oscillations and sensorimotor performance.Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3863-8. doi: 10.1073/pnas.0407920102. Epub 2005 Feb 28. Proc Natl Acad Sci U S A. 2005. PMID: 15738424 Free PMC article.
-
A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task.J Neurophysiol. 2010 Aug;104(2):829-39. doi: 10.1152/jn.00166.2010. Epub 2010 Jun 10. J Neurophysiol. 2010. PMID: 20538778 Free PMC article.
-
Circuit oscillations in odor perception and memory.Prog Brain Res. 2014;208:223-51. doi: 10.1016/B978-0-444-63350-7.00009-7. Prog Brain Res. 2014. PMID: 24767485 Review.
-
Odor perception and olfactory bulb plasticity in adult mammals.J Neurophysiol. 2009 May;101(5):2204-9. doi: 10.1152/jn.00076.2009. Epub 2009 Mar 4. J Neurophysiol. 2009. PMID: 19261715 Review.
Cited by
-
Gamma and Beta Oscillations Define a Sequence of Neurocognitive Modes Present in Odor Processing.J Neurosci. 2016 Jul 20;36(29):7750-67. doi: 10.1523/JNEUROSCI.0569-16.2016. J Neurosci. 2016. PMID: 27445151 Free PMC article.
-
Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks?Front Behav Neurosci. 2014 Jun 23;8:218. doi: 10.3389/fnbeh.2014.00218. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25002840 Free PMC article. Review.
-
Granule cell excitability regulates gamma and beta oscillations in a model of the olfactory bulb dendrodendritic microcircuit.J Neurophysiol. 2016 Aug 1;116(2):522-39. doi: 10.1152/jn.00988.2015. Epub 2016 Apr 27. J Neurophysiol. 2016. PMID: 27121582 Free PMC article.
-
Odor Enrichment Increases Hippocampal Neuron Numbers in Mouse.Exp Neurobiol. 2018 Apr;27(2):94-102. doi: 10.5607/en.2018.27.2.94. Epub 2018 Apr 26. Exp Neurobiol. 2018. PMID: 29731675 Free PMC article.
-
Changes in pairwise correlations during running reshape global network state in the main olfactory bulb.J Neurophysiol. 2021 May 1;125(5):1612-1623. doi: 10.1152/jn.00464.2020. Epub 2021 Mar 3. J Neurophysiol. 2021. PMID: 33656931 Free PMC article.
References
-
- Akaike H. Fitting autoregressive models for prediction. Ann Inst Statist Math 21: 243, 1969.
-
- Albo Z, Di Prisco G, Chen Y, Rangarajan G, Truccolo W, Feng J, Vertes R, Ding M. Is partial coherence a viable technique for identifying generators of neural oscillations? Biol Cybern 90: 318–326, 2004. - PubMed
-
- Amaral D, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591, 1989. - PubMed
-
- Ashley R, Granger CWJ, Schmalensee R. Advertising and aggregate consumption: an analysis of causality. Econometrica 48: 1149–1168, 1980.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

