Mapping human somatosensory cortex in individual subjects with 7T functional MRI
- PMID: 20164393
- PMCID: PMC2867563
- DOI: 10.1152/jn.01017.2009
Mapping human somatosensory cortex in individual subjects with 7T functional MRI
Abstract
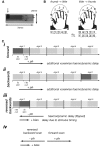
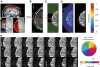
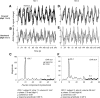
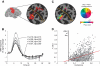
Functional magnetic resonance imaging (fMRI) is now routinely used to map the topographic organization of human visual cortex. Mapping the detailed topography of somatosensory cortex, however, has proven to be more difficult. Here we used the increased blood-oxygen-level-dependent contrast-to-noise ratio at ultra-high field (7 Tesla) to measure the topographic representation of the digits in human somatosensory cortex at 1 mm isotropic resolution in individual subjects. A "traveling wave" paradigm was used to locate regions of cortex responding to periodic tactile stimulation of each distal phalangeal digit. Tactile stimulation was applied sequentially to each digit of the left hand from thumb to little finger (and in the reverse order). In all subjects, we found an orderly map of the digits on the posterior bank of the central sulcus (postcentral gyrus). Additionally, we measured event-related responses to brief stimuli for comparison with the topographic mapping data and related the fMRI responses to anatomical images obtained with an inversion-recovery sequence. Our results have important implications for the study of human somatosensory cortex and underscore the practical utility of ultra-high field functional imaging with 1 mm isotropic resolution for neuroscience experiments. First, topographic mapping of somatosensory cortex can be achieved in 20 min, allowing time for further experiments in the same session. Second, the maps are of sufficiently high resolution to resolve the representations of all five digits and third, the measurements are robust and can be made in an individual subject. These combined advantages will allow somatotopic fMRI to be used to measure the representation of digits in patients undergoing rehabilitation or plastic changes after peripheral nerve damage as well as tracking changes in normal subjects undergoing perceptual learning.
Figures


Similar articles
-
Development of a multichannel hand-adaptive tactile stimulation device for somatotopic map of human hand in somatosensory cortex with fMRI.Neuroimage. 2025 Apr 15;310:121126. doi: 10.1016/j.neuroimage.2025.121126. Epub 2025 Mar 6. Neuroimage. 2025. PMID: 40057288
-
Individual left-hand and right-hand intra-digit representations in human primary somatosensory cortex.Eur J Neurosci. 2015 Sep;42(5):2155-63. doi: 10.1111/ejn.12978. Epub 2015 Jul 15. Eur J Neurosci. 2015. PMID: 26061413
-
Distinct fine-scale fMRI activation patterns of contra- and ipsilateral somatosensory areas 3b and 1 in humans.Hum Brain Mapp. 2014 Sep;35(9):4841-57. doi: 10.1002/hbm.22517. Epub 2014 Apr 1. Hum Brain Mapp. 2014. PMID: 24692215 Free PMC article.
-
From maps to form to space: touch and the body schema.Neuropsychologia. 2010 Feb;48(3):645-54. doi: 10.1016/j.neuropsychologia.2009.08.017. Epub 2009 Aug 20. Neuropsychologia. 2010. PMID: 19699214 Free PMC article. Review.
-
Exploring structure and function of sensory cortex with 7T MRI.Neuroimage. 2018 Jan 1;164:10-17. doi: 10.1016/j.neuroimage.2017.01.081. Epub 2017 Feb 2. Neuroimage. 2018. PMID: 28161312 Review.
Cited by
-
Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus.Hum Brain Mapp. 2014 Jan;35(1):213-26. doi: 10.1002/hbm.22172. Epub 2012 Sep 11. Hum Brain Mapp. 2014. PMID: 22965769 Free PMC article.
-
Brain Encoding of Naturalistic, Continuous, and Unpredictable Tactile Events.eNeuro. 2024 Sep 24;11(9):ENEURO.0238-24.2024. doi: 10.1523/ENEURO.0238-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39266328 Free PMC article.
-
The functional architecture of S1 during touch observation described with 7 T fMRI.Brain Struct Funct. 2014 Jan;219(1):119-40. doi: 10.1007/s00429-012-0489-z. Epub 2013 Jan 3. Brain Struct Funct. 2014. PMID: 23283478 Free PMC article.
-
Consistency and stability of individualized cortical functional networks parcellation at 3.0 T and 5.0 T MRI.Front Neurosci. 2024 Aug 19;18:1425032. doi: 10.3389/fnins.2024.1425032. eCollection 2024. Front Neurosci. 2024. PMID: 39224574 Free PMC article.
-
Fine-Grained Mapping of Cortical Somatotopies in Chronic Complex Regional Pain Syndrome.J Neurosci. 2019 Nov 13;39(46):9185-9196. doi: 10.1523/JNEUROSCI.2005-18.2019. Epub 2019 Sep 30. J Neurosci. 2019. PMID: 31570533 Free PMC article.
References
-
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron 20: 285–296, 1998. - PubMed
-
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739, 1998. - PubMed
-
- Butterworth S, Francis S, Kelly E, McGlone F, Bowtell R, Sawle GV. Abnormal cortical sensory activation in dystonia: an fMRI study. Mov Disord 18: 673–682, 2003. - PubMed
-
- Darian-Smith I. Touch in primates. Annu Rev Psychol 33: 155–194, 1982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

