CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections
- PMID: 20164414
- PMCID: PMC3253758
- DOI: 10.4049/jimmunol.0902168
CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections
Abstract
In this study, we demonstrate complex networks of CD8 T cell cross-reactivities between influenza A virus and EBV in humans and between lymphocytic choriomeningitis virus and vaccinia virus in mice. We also show directly that cross-reactive T cells mediate protective heterologous immunity in mice. Subsets of T cell populations reactive with one epitope cross-reacted with either of several other epitopes encoded by the same or the heterologous virus. Human T cells specific to EBV-encoded BMLF1(280-288) could be cross-reactive with two influenza A virus or two other EBV epitopes. Mouse T cells specific to the vaccinia virus-encoded a11r(198-205) could be cross-reactive with three different lymphocytic choriomeningitis virus, one Pichinde virus, or one other vaccinia virus epitope. Patterns of cross-reactivity differed among individuals, reflecting the private specificities of the host's immune repertoire and divergence in the abilities of T cell populations to mediate protective immunity. Defining such cross-reactive networks between commonly encountered human pathogens may facilitate the design of vaccines.
Figures



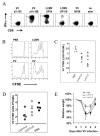
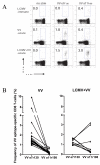
 ). VV titers in the testes were assayed on day 4 post-infection (LV/a11r-3 vs. PBS: p<0.01; L/a11r-2 vs. PBS: p<0.05). (D) Differential effect on VV titers upon the use of different cross-reactive a11r-specific CD8 T-cell lines, L/a11r-4 and L/a11r-5. There was a greater reduction of VV titers in testes day 4 post-infection in mice injected with L/a11r-4 (
). VV titers in the testes were assayed on day 4 post-infection (LV/a11r-3 vs. PBS: p<0.01; L/a11r-2 vs. PBS: p<0.05). (D) Differential effect on VV titers upon the use of different cross-reactive a11r-specific CD8 T-cell lines, L/a11r-4 and L/a11r-5. There was a greater reduction of VV titers in testes day 4 post-infection in mice injected with L/a11r-4 ( ) compared to L/a11r-5 (엯)(p<0.08, n=4), or PBS (●)(p<0.08, n=4). (E) Time course of weight loss after VV infection. Adoptive transfer of the T-cell line L/a11r-4 resulted in a 50% inhibition of weight loss (day 2: 2.4%±0.6% vs. 4.8%±0.7% weight loss, n=4, p=0.06; day 3: 2.3%±0.5% vs. 4.5%±0.7% weight loss, n=4, p=0.08) post VV-infection, while T-cell line L/a11r-5 did not affect weight loss when compared to the control, PBS injected, mice (p>0.3).
) compared to L/a11r-5 (엯)(p<0.08, n=4), or PBS (●)(p<0.08, n=4). (E) Time course of weight loss after VV infection. Adoptive transfer of the T-cell line L/a11r-4 resulted in a 50% inhibition of weight loss (day 2: 2.4%±0.6% vs. 4.8%±0.7% weight loss, n=4, p=0.06; day 3: 2.3%±0.5% vs. 4.5%±0.7% weight loss, n=4, p=0.08) post VV-infection, while T-cell line L/a11r-5 did not affect weight loss when compared to the control, PBS injected, mice (p>0.3).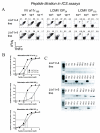
Similar articles
-
Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation.J Immunol. 2002 Jul 1;169(1):90-8. doi: 10.4049/jimmunol.169.1.90. J Immunol. 2002. PMID: 12077233
-
Severity of Acute Infectious Mononucleosis Correlates with Cross-Reactive Influenza CD8 T-Cell Receptor Repertoires.mBio. 2017 Dec 5;8(6):e01841-17. doi: 10.1128/mBio.01841-17. mBio. 2017. PMID: 29208744 Free PMC article.
-
Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections.J Immunol. 2003 Apr 15;170(8):4077-86. doi: 10.4049/jimmunol.170.8.4077. J Immunol. 2003. PMID: 12682237
-
Evolution of the CD8 T-cell repertoire during infections.Microbes Infect. 2000 Jul;2(9):1025-39. doi: 10.1016/s1286-4579(00)01257-0. Microbes Infect. 2000. PMID: 10967283 Review.
-
Heterologous immunity between viruses.Immunol Rev. 2010 May;235(1):244-66. doi: 10.1111/j.0105-2896.2010.00897.x. Immunol Rev. 2010. PMID: 20536568 Free PMC article. Review.
Cited by
-
Heterologous Immunity and Persistent Murine Cytomegalovirus Infection.J Virol. 2017 Jan 3;91(2):e01386-16. doi: 10.1128/JVI.01386-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807227 Free PMC article.
-
Allo-priming as a universal anti-viral vaccine: protecting elderly from current COVID-19 and any future unknown viral outbreak.J Transl Med. 2020 May 12;18(1):196. doi: 10.1186/s12967-020-02363-3. J Transl Med. 2020. PMID: 32398026 Free PMC article.
-
Cross-reactivity influences changes in human influenza A virus and Epstein Barr virus specific CD8 memory T cell receptor alpha and beta repertoires between young and old.Front Immunol. 2023 Feb 24;13:1011935. doi: 10.3389/fimmu.2022.1011935. eCollection 2022. Front Immunol. 2023. PMID: 36923729 Free PMC article.
-
Cross-reactive responses to modified M1₅₈-₆₆ peptides by CD8⁺ T cells that use noncanonical BV genes can describe unknown repertoires.Eur J Immunol. 2012 Nov;42(11):3001-8. doi: 10.1002/eji.201242596. Epub 2012 Sep 20. Eur J Immunol. 2012. PMID: 22865108 Free PMC article.
-
Increased Immune Response Variability during Simultaneous Viral Coinfection Leads to Unpredictability in CD8 T Cell Immunity and Pathogenesis.J Virol. 2015 Nov;89(21):10786-801. doi: 10.1128/JVI.01432-15. Epub 2015 Aug 12. J Virol. 2015. PMID: 26269191 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI-46578/AI/NIAID NIH HHS/United States
- AI-42845/AI/NIAID NIH HHS/United States
- 5 T32 AI-07349-16/AI/NIAID NIH HHS/United States
- R01 AI046578/AI/NIAID NIH HHS/United States
- P30 AI042845/AI/NIAID NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- P01 AI049320/AI/NIAID NIH HHS/United States
- AI-35506/AI/NIAID NIH HHS/United States
- AI-49320/AI/NIAID NIH HHS/United States
- AI-054455/AI/NIAID NIH HHS/United States
- DR-32520/PHS HHS/United States
- T32 AI007349/AI/NIAID NIH HHS/United States
- R01 AI081675/AI/NIAID NIH HHS/United States
- R01 AR035506/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

