Transfusion of nonobese diabetic mice with allogeneic newborn blood ameliorates autoimmune diabetes and modifies the expression of selected immune response genes
- PMID: 20164427
- PMCID: PMC3779363
- DOI: 10.4049/jimmunol.0903615
Transfusion of nonobese diabetic mice with allogeneic newborn blood ameliorates autoimmune diabetes and modifies the expression of selected immune response genes
Abstract
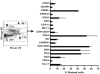
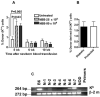
Although allogeneic bone marrow transplantation has been shown to prevent autoimmune diabetes in heavily irradiated nonobese diabetic (NOD) mice, a similar procedure is not suitable for the treatment of patients with type 1 diabetes because of associated severe side effects. Therefore, we evaluated whether mouse newborn blood (NBB), equivalent to human umbilical cord blood, could be used for diabetes prevention without recipient preconditioning. To test this hypothesis, unconditioned, prediabetic female NOD mice were given a single injection of whole NBB derived from the allogeneic diabetes-resistant mouse strain C57BL/6. Transfusion of allogeneic NBB but not adult blood prevented diabetes incidence in a majority of treated mice for a prolonged period of time. This was accompanied by the release of insulin in response to a challenge with glucose. Invasive cellular infiltration of islets was also substantially reduced in these mice. Although NBB transfusion induced a low level of hematopoietic microchimerism, it did not strictly correlate with amelioration of diabetes. Induction of genes implicated in diabetes, such as Il18, Tnfa, and Inos but not Il4, Il17 or Ifng, was repressed in splenocytes derived from protected mice. Notably, expression of the transcription factor Tbet/Tbx21 but not Gata3 or Rorgt was upregulated in protected mice. These data indicate that allogeneic NBB transfusion can prevent diabetes in NOD mice associated with modulation of selected cytokine genes implicated in diabetes manifestation. The data presented in this study provide the proof of principle for the utility of allogeneic umbilical cord blood transfusion to treat patients with autoimmune diabetes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
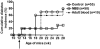
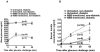

Similar articles
-
Chromatin remodeling resets the immune system to protect against autoimmune diabetes in mice.Immunol Cell Biol. 2011 Jul;89(5):640-9. doi: 10.1038/icb.2010.144. Epub 2011 Feb 15. Immunol Cell Biol. 2011. PMID: 21321581
-
Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in nonobese diabetic (NOD) mice.J Immunol. 1996 Jan 1;156(1):380-8. J Immunol. 1996. PMID: 8598488
-
Patterns of hemopoietic reconstitution in nonobese diabetic mice: dichotomy of allogeneic resistance versus competitive advantage of disease-resistant marrow.J Immunol. 1997 Mar 1;158(5):2435-42. J Immunol. 1997. PMID: 9036994
-
Type I diabetes mellitus: a predictable autoimmune disease with interindividual variation in the rate of beta cell destruction.Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 2):S85-95. doi: 10.1016/0090-1229(89)90115-3. Clin Immunol Immunopathol. 1989. PMID: 2642771 Review.
-
Progress toward production of immunologic tolerance with no or minimal toxic immunosuppression for prevention of immunodeficiency and autoimmune diseases.World J Surg. 2000 Jul;24(7):797-810. doi: 10.1007/s002680010128. World J Surg. 2000. PMID: 10833246 Review.
Cited by
-
Neonatal umbilical cord blood transplantation halts skeletal disease progression in the murine model of MPS-I.Sci Rep. 2017 Aug 25;7(1):9473. doi: 10.1038/s41598-017-09958-9. Sci Rep. 2017. PMID: 28842642 Free PMC article.
-
Y-chromosome DNA is present in the blood of female dogs suggesting the presence of fetal microchimerism.PLoS One. 2013 Jul 8;8(7):e68114. doi: 10.1371/journal.pone.0068114. Print 2013. PLoS One. 2013. PMID: 23861856 Free PMC article.
-
Treatment of autoimmune encephalomyelitis with a histone deacetylase inhibitor.Free Neuropathol. 2020 Jul 14;1:19. doi: 10.17879/freeneuropathology-2020-2819. eCollection 2020 Jan. Free Neuropathol. 2020. PMID: 37283668 Free PMC article.
-
An update on the use of NOD mice to study autoimmune (Type 1) diabetes.Expert Rev Clin Immunol. 2010 Nov;6(6):939-55. doi: 10.1586/eci.10.68. Expert Rev Clin Immunol. 2010. PMID: 20979558 Free PMC article.
-
Transcriptome analysis of epigenetically modulated genome indicates signature genes in manifestation of type 1 diabetes and its prevention in NOD mice.PLoS One. 2013;8(1):e55074. doi: 10.1371/journal.pone.0055074. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383062 Free PMC article.
References
-
- Eisenbarth GS. Update in type 1 diabetes. J Clin Endocrinol Metab. 2007;92:2403–2407. - PubMed
-
- Leiter EH. Nonobese diabetic mice and the genetics of diabetes susceptibility. Curr Diab Rep. 2005;5:141–148. - PubMed
-
- LaFace DM, Peck AB. Reciprocal allogeneic bone marrow transplantation between NOD mice and diabetes-nonsusceptible mice associated with transfer and prevention of autoimmune diabetes. Diabetes. 1989;38:894–901. - PubMed
-
- Li H, Kaufman CL, Ildstad ST. Allogeneic chimerism induces donor-specific tolerance to simultaneous islet allografts in nonobese diabetic mice. Surgery. 1995;118:192–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

