Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling
- PMID: 20164444
- PMCID: PMC2845418
- DOI: 10.1105/tpc.109.071225
Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling
Abstract
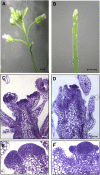
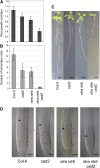
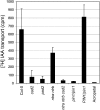
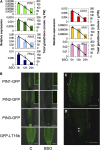
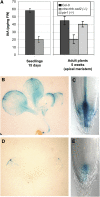
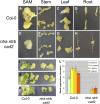
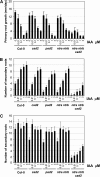
Intracellular redox status is a critical parameter determining plant development in response to biotic and abiotic stress. Thioredoxin (TRX) and glutathione are key regulators of redox homeostasis, and the TRX and glutathione pathways are essential for postembryonic meristematic activities. Here, we show by associating TRX reductases (ntra ntrb) and glutathione biosynthesis (cad2) mutations that these two thiol reduction pathways interfere with developmental processes through modulation of auxin signaling. The triple ntra ntrb cad2 mutant develops normally at the rosette stage, undergoes the floral transition, but produces almost naked stems, reminiscent of the phenotype of several mutants affected in auxin transport or biosynthesis. In addition, the ntra ntrb cad2 mutant shows a loss of apical dominance, vasculature defects, and reduced secondary root production, several phenotypes tightly regulated by auxin. We further show that auxin transport capacities and auxin levels are perturbed in the mutant, suggesting that the NTR-glutathione pathways alter both auxin transport and metabolism. Analysis of ntr and glutathione biosynthesis mutants suggests that glutathione homeostasis plays a major role in auxin transport as both NTR and glutathione pathways are involved in auxin homeostasis.
Figures


Comment in
-
Redox regulation of auxin signaling and plant development.Plant Cell. 2010 Feb;22(2):295. doi: 10.1105/tpc.110.220212. Epub 2010 Feb 17. Plant Cell. 2010. PMID: 20164443 Free PMC article. No abstract available.
References
-
- Arnér E.S., Holmgren A. (2000). Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267: 6102–6109 - PubMed
-
- Ball L., Accotto G., Bechtold U., Creissen G., Funck D., Jimenez A., Kular B., Leyland N., Mejia-Carranza J., Reynolds H., Karpinski S., Mullineaux P.M. (2004). Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

