Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration
- PMID: 20164570
- PMCID: PMC2922983
- DOI: 10.3233/JAD-2010-1375
Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration
Abstract
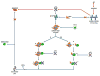
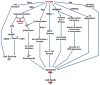
Recently, the oxidoreductase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), has become a subject of interest as more and more studies reveal a surfeit of diverse GAPDH functions, extending beyond traditional aerobic metabolism of glucose. As a result of multiple isoforms and cellular locales, GAPDH is able to come in contact with a variety of small molecules, proteins, membranes, etc., that play important roles in normal and pathologic cell function. Specifically, GAPDH has been shown to interact with neurodegenerative disease-associated proteins, including the amyloid-beta protein precursor (AbetaPP). Studies from our laboratory have shown significant inhibition of GAPDH dehydrogenase activity in Alzheimer's disease (AD) brain due to oxidative modification. Although oxidative stress and damage is a common phenomenon in the AD brain, it would seem that inhibition of glycolytic enzyme activity is merely one avenue in which AD pathology affects neuronal cell development and survival, as oxidative modification can also impart a toxic gain-of-function to many proteins, including GAPDH. In this review, we examine the many functions of GAPDH with respect to AD brain; in particular, the apparent role(s) of GAPDH in AD-related apoptotic cell death is emphasized.
Figures

References
-
- Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–184. - PubMed
-
- Jenkins JL, Tanner JJ. High-resolution structure of human D-glyceraldehyde-3-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr. 2006;62:290–301. - PubMed
-
- Branlant G, Branlant C. Nucleotide sequence of the Escherichia coli gap gene. Different evolutionary behavior of the NAD+-binding domain and of the catalytic domain of D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1985;150:61–66. - PubMed
-
- Sirover MA. Role of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in normal cell function and in cell pathology. J Cell Biochem. 1997;66:133–140. - PubMed
-
- Baxi MD, Vishwanatha JK. Uracil DNA-glycosylase/glyceraldehyde-3-phosphate dehydrogenase is an Ap4A binding protein. Biochemistry. 1995;34:9700–9707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

