Triple-negative breast cancer: role of specific chemotherapy agents
- PMID: 20164691
- PMCID: PMC2882502
- DOI: 10.1097/PPO.0b013e3181d24ff7
Triple-negative breast cancer: role of specific chemotherapy agents
Abstract
Cytotoxic chemotherapy remains the mainstay of treatment for triple-negative breast cancer (TNBC) despite the promise of new targeted and biologic agents. Many studies have shown significant benefit of chemotherapy in the neoadjuvant, adjuvant, and metastatic treatment of TNBC. Neoadjuvant chemotherapy studies have consistently reported higher response rates in TNBC than non-TNBC, and pathologic complete response has been shown to predict improved long-term outcomes for TNBC. Although the specific adjuvant regimens that may be most effective for TNBC are still being determined, third-generation chemotherapy regimens using dose dense or metronomic polychemotherapy are among the most effective tools presently available. The role of specific chemotherapy agents in the treatment of TNBC remains incompletely defined and warrants careful review to ensure that the most effective therapy is delivered while minimizing unnecessary toxicity. Platinum agents have seen renewed interest in TNBC based on a growing body of preclinical and clinical data suggesting encouraging activity. Taxanes and anthracyclines are active in TNBC and remain important agents but have not shown specific benefit over non-TNBC. Capecitabine has limited reported data in TNBC, but some reports suggest differential activity in TNBC compared with hormone receptor-positive breast cancer. TNBC is itself a heterogeneous group in which subgroups such as BRCA1 mutation carriers may have particular sensitivity to platinum agents and relatively less sensitivity to taxanes. Therefore, the identification of additional molecular biomarkers to predict response to specific chemotherapy is required to further improve treatment strategies with the current menu of chemotherapy options and future combinations with targeted therapies.
Figures
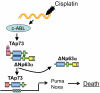
References
-
- Irvin WJ, Jr., Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44(18):2799–805. - PubMed
-
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clinical breast cancer. 2009;9(1):29–33. - PubMed
-
- Sledge G, Miller K, Moisa C, Gradishar W. Safety and efficacy of capecitabine (C) plus bevacizumab (B) as first-line in metastatic breast cancer. J Clin Oncol. 2007 June 20;Vol 25(No. 18S):1013. ASCO Annual Meeting Proceedings Part I. Supplement.
-
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

