Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa
- PMID: 20164818
- PMCID: PMC2855750
- DOI: 10.1097/TP.0b013e3181d45a61
Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa
Abstract
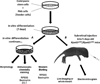
Background: To study whether C57BL/6J-Tyr/J (C2J) mouse embryonic stem (ES) cells can differentiate into retinal pigment epithelial (RPE) cells in vitro and then restore retinal function in a model for retinitis pigmentosa: Rpe65/Rpe65 C57BL6 mice.
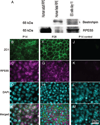
Methods: Yellow fluorescent protein (YFP)-labeled C2J ES cells were induced to differentiate into RPE-like structures on PA6 feeders. RPE-specific markers are expressed from differentiated cells in vitro. After differentiation, ES cell-derived RPE-like cells were transplanted into the subretinal space of postnatal day 5 Rpe65/Rpe65 mice. Live imaging of YFP-labeled C2J ES cells demonstrated survival of the graft. Electroretinograms (ERGs) were performed on transplanted mice to evaluate the functional outcome of transplantation.
Results: RPE-like cells derived from ES cells sequentially express multiple RPE-specific markers. After transplantation, YFP-labeled cells can be tracked with live imaging for as long as 7 months. Although more than half of the mice were complicated with retinal detachments or tumor development, one fourth of the mice showed increased electroretinogram responses in the transplanted eyes. Rpe65/Rpe65 mice transplanted with RPE-like cells showed significant visual recovery during a 7-month period, whereas those injected with saline, PA6 feeders, or undifferentiated ES cells showed no rescue.
Conclusions: ES cells can differentiate, morphologically, and functionally, into RPE-like cells. Based on these findings, differentiated ES cells have the potential for the development of new therapeutic approaches for RPE-specific diseases such as certain forms of retinitis pigmentosa and macular degeneration. Nevertheless, stringent control of retinal detachment and teratoma development will be necessary before initiation of treatment trials.
Figures




References
-
- Haruta M, Sasai Y, Kawasaki H, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004;45:1020. - PubMed
-
- Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004;23:149. - PubMed
-
- Haruta M. Embryonic stem cells: Potential source for ocular repair. Semin Ophthalmol. 2005;20:17. - PubMed
-
- Aoki H, Hara A, Nakagawa S, et al. Embryonic stem cells that differentiate into RPE cell precursors in vitro develop into RPE cell monolayers in vivo. Exp Eye Res. 2006;82:265. - PubMed
-
- Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

