Lack of humoral immune response to the tetracycline (Tet) activator in rats injected intracranially with Tet-off rAAV vectors
- PMID: 20164859
- PMCID: PMC2869394
- DOI: 10.1038/gt.2010.6
Lack of humoral immune response to the tetracycline (Tet) activator in rats injected intracranially with Tet-off rAAV vectors
Abstract
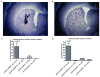
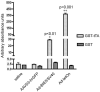
The ability to safely control transgene expression from viral vectors is a long-term goal in the gene therapy field. We have previously reported tight regulation of GFP expression in rat brain using a self-regulating tet-off rAAV vector. The immune responses against tet regulatory elements observed by other groups in nonhuman primates after intramuscular injection of tet-on encoding vectors raise concerns about the clinical value of tet-regulated vectors. However, previous studies have not examined immune responses following injection of AAV vectors into brain. Therefore, rat striatum was injected with tet-off rAAV harboring a therapeutic gene for Parkinson's disease, either hAADC or hGDNF. The expression of each gene was tightly controlled by the tet-off regulatory system. Using an ELISA developed with purified GST-tTA protein, no detectable immunogenicity against tTA was observed in sera of rats that received an intrastriatal injection of either vector. In contrast, sera from rats intradermally injected with an adenovirus containing either tTA or rtTA, as positive controls, had readily detectable antibodies. These observations suggest that tet-off rAAV vectors do not elicit an immune response when injected into rat brain and that these may offer safer vectors for Parkinson's disease than vectors with constitutive expression.
Conflict of interest statement
Figures

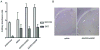
Similar articles
-
Tight regulation from a single tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR.Gene Ther. 2004 Jul;11(13):1057-67. doi: 10.1038/sj.gt.3302245. Gene Ther. 2004. PMID: 15152187
-
Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus.J Virol. 2002 Nov;76(22):11605-11. doi: 10.1128/jvi.76.22.11605-11611.2002. J Virol. 2002. PMID: 12388721 Free PMC article.
-
Tightly regulated long-term erythropoietin expression in vivo using tet-inducible recombinant adeno-associated viral vectors.Hum Gene Ther. 2002 Jan 20;13(2):335-42. doi: 10.1089/10430340252769842. Hum Gene Ther. 2002. PMID: 11812288
-
[Advances in the application of gene therapy for Parkinson's disease with adeno-associated virus].Yao Xue Xue Bao. 2014 May;49(5):576-81. Yao Xue Xue Bao. 2014. PMID: 25151724 Review. Chinese.
-
[Gene therapy for Parkinson's disease].Nihon Rinsho. 2005 Dec;63 Suppl 12:622-5. Nihon Rinsho. 2005. PMID: 16416863 Review. Japanese. No abstract available.
Cited by
-
Gene regulation systems for gene therapy applications in the central nervous system.Neurol Res Int. 2012;2012:595410. doi: 10.1155/2012/595410. Epub 2012 Jan 5. Neurol Res Int. 2012. PMID: 22272373 Free PMC article.
-
Retrograde labeling, transduction, and genetic targeting allow cellular analysis of corticospinal motor neurons: implications in health and disease.Front Neuroanat. 2014 Mar 26;8:16. doi: 10.3389/fnana.2014.00016. eCollection 2014. Front Neuroanat. 2014. PMID: 24723858 Free PMC article. Review.
-
Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety.Int J Mol Sci. 2018 Nov 3;19(11):3455. doi: 10.3390/ijms19113455. Int J Mol Sci. 2018. PMID: 30400287 Free PMC article.
-
Genetically engineered suicide gene in mesenchymal stem cells using a Tet-On system for anaplastic thyroid cancer.PLoS One. 2017 Jul 20;12(7):e0181318. doi: 10.1371/journal.pone.0181318. eCollection 2017. PLoS One. 2017. PMID: 28727740 Free PMC article.
-
Production and titering of recombinant adeno-associated viral vectors.J Vis Exp. 2011 Nov 27;(57):e3348. doi: 10.3791/3348. J Vis Exp. 2011. PMID: 22143312 Free PMC article.
References
-
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. - PubMed
-
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. - PubMed
-
- Sanchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic L-amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther. 2001;4:324–330. - PubMed
-
- Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–275. - PubMed
-
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

