Active site remodelling accompanies thioester bond formation in the SUMO E1
- PMID: 20164921
- PMCID: PMC2866016
- DOI: 10.1038/nature08765
Active site remodelling accompanies thioester bond formation in the SUMO E1
Abstract
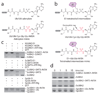
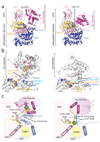
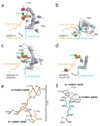
E1 enzymes activate ubiquitin (Ub) and ubiquitin-like (Ubl) proteins in two steps by carboxy-terminal adenylation and thioester bond formation to a conserved catalytic cysteine in the E1 Cys domain. The structural basis for these intermediates remains unknown. Here we report crystal structures for human SUMO E1 in complex with SUMO adenylate and tetrahedral intermediate analogues at 2.45 and 2.6 A, respectively. These structures show that side chain contacts to ATP.Mg are released after adenylation to facilitate a 130 degree rotation of the Cys domain during thioester bond formation that is accompanied by remodelling of key structural elements including the helix that contains the E1 catalytic cysteine, the crossover and re-entry loops, and refolding of two helices that are required for adenylation. These changes displace side chains required for adenylation with side chains required for thioester bond formation. Mutational and biochemical analyses indicate these mechanisms are conserved in other E1s.
Figures



Comment in
-
Structural biology: Transformative encounters.Nature. 2010 Feb 18;463(7283):889-90. doi: 10.1038/463889a. Nature. 2010. PMID: 20164915 No abstract available.
-
And yet it moves: active site remodeling in the SUMO E1.Structure. 2010 Mar 14;18(4):419-21. doi: 10.1016/j.str.2010.03.005. Structure. 2010. PMID: 20399179
Similar articles
-
Domain alternation and active site remodeling are conserved structural features of ubiquitin E1.J Biol Chem. 2017 Jul 21;292(29):12089-12099. doi: 10.1074/jbc.M117.787622. Epub 2017 Jun 1. J Biol Chem. 2017. PMID: 28572513 Free PMC article.
-
Structural basis for adenylation and thioester bond formation in the ubiquitin E1.Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15475-15484. doi: 10.1073/pnas.1905488116. Epub 2019 Jun 24. Proc Natl Acad Sci U S A. 2019. PMID: 31235585 Free PMC article.
-
Structures of the SUMO E1 provide mechanistic insights into SUMO activation and E2 recruitment to E1.EMBO J. 2005 Feb 9;24(3):439-51. doi: 10.1038/sj.emboj.7600552. Epub 2005 Jan 20. EMBO J. 2005. PMID: 15660128 Free PMC article.
-
Chemical Tools for Probing the Ub/Ubl Conjugation Cascades.Chembiochem. 2025 Jan 2;26(1):e202400659. doi: 10.1002/cbic.202400659. Epub 2024 Nov 6. Chembiochem. 2025. PMID: 39313481 Free PMC article. Review.
-
Protein interactions in the sumoylation cascade: lessons from X-ray structures.FEBS J. 2008 Jun;275(12):3003-15. doi: 10.1111/j.1742-4658.2008.06459.x. Epub 2008 May 17. FEBS J. 2008. PMID: 18492068 Review.
Cited by
-
Targeting SUMOylation dependency in human cancer stem cells through a unique SAE2 motif revealed by chemical genomics.Cell Chem Biol. 2021 Oct 21;28(10):1394-1406.e10. doi: 10.1016/j.chembiol.2021.04.014. Epub 2021 May 11. Cell Chem Biol. 2021. PMID: 33979648 Free PMC article.
-
Mechanistic insight into protein modification and sulfur mobilization activities of noncanonical E1 and associated ubiquitin-like proteins of Archaea.FEBS J. 2016 Oct;283(19):3567-3586. doi: 10.1111/febs.13819. FEBS J. 2016. PMID: 27459543 Free PMC article.
-
An E1-E2 fusion protein primes antiviral immune signalling in bacteria.Nature. 2023 Apr;616(7956):319-325. doi: 10.1038/s41586-022-05647-4. Epub 2023 Feb 8. Nature. 2023. PMID: 36755092 Free PMC article.
-
SUMOylation in the control of cholesterol homeostasis.Open Biol. 2020 May;10(5):200054. doi: 10.1098/rsob.200054. Epub 2020 May 6. Open Biol. 2020. PMID: 32370667 Free PMC article. Review.
-
The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition.Nat Rev Mol Cell Biol. 2010 Dec;11(12):861-71. doi: 10.1038/nrm3011. Nat Rev Mol Cell Biol. 2010. PMID: 21102611 Free PMC article. Review.
References
-
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. - PubMed
-
- Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Laney JD, Hochstrasser M. Substrate targeting in the ubiquitin system. Cell. 1999;97(4):427–430. - PubMed
-
- Dye BT, Schulman BA. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 2007;36:131–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

