TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity
- PMID: 20164930
- PMCID: PMC3273423
- DOI: 10.1038/nature08746
TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity
Abstract
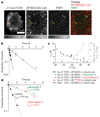
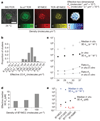
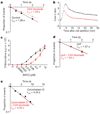
The recognition of foreign antigens by T lymphocytes is essential to most adaptive immune responses. It is driven by specific T-cell antigen receptors (TCRs) binding to antigenic peptide-major histocompatibility complex (pMHC) molecules on other cells. If productive, these interactions promote the formation of an immunological synapse. Here we show that synaptic TCR-pMHC binding dynamics differ significantly from TCR-pMHC binding in solution. We used single-molecule microscopy and fluorescence resonance energy transfer (FRET) between fluorescently tagged TCRs and their cognate pMHC ligands to measure the kinetics of TCR-pMHC binding in situ. When compared with solution measurements, the dissociation of this complex was increased significantly (4-12-fold). Disruption of actin polymers reversed this effect, indicating that cytoskeletal dynamics destabilize this interaction directly or indirectly. Nevertheless, TCR affinity for pMHC was significantly elevated as the result of a large (about 100-fold) increase in the association rate, a likely consequence of complementary molecular orientation and clustering. In helper T cells, the CD4 molecule has been proposed to bind cooperatively with the TCR to the same pMHC complex. However, CD4 blockade had no effect on the synaptic TCR affinity, nor did it destabilize TCR-pMHC complexes, indicating that the TCR binds pMHC independently of CD4.
Figures

Similar articles
-
Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse.Nature. 2014 Mar 6;507(7490):118-23. doi: 10.1038/nature12951. Epub 2014 Feb 2. Nature. 2014. PMID: 24487619 Free PMC article.
-
Force-Regulated In Situ TCR-Peptide-Bound MHC Class II Kinetics Determine Functions of CD4+ T Cells.J Immunol. 2015 Oct 15;195(8):3557-64. doi: 10.4049/jimmunol.1501407. Epub 2015 Sep 2. J Immunol. 2015. PMID: 26336148 Free PMC article.
-
Förster Resonance Energy Transfer to Study TCR-pMHC Interactions in the Immunological Synapse.Methods Mol Biol. 2017;1584:207-229. doi: 10.1007/978-1-4939-6881-7_14. Methods Mol Biol. 2017. PMID: 28255705
-
Modulation of T cell function by TCR/pMHC binding kinetics.Immunobiology. 2006;211(1-2):47-64. doi: 10.1016/j.imbio.2005.09.003. Epub 2006 Jan 4. Immunobiology. 2006. PMID: 16446170 Review.
-
How TCRs bind MHCs, peptides, and coreceptors.Annu Rev Immunol. 2006;24:419-66. doi: 10.1146/annurev.immunol.23.021704.115658. Annu Rev Immunol. 2006. PMID: 16551255 Review.
Cited by
-
The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility.J Cell Biol. 2015 Feb 16;208(4):457-73. doi: 10.1083/jcb.201406120. Epub 2015 Feb 9. J Cell Biol. 2015. PMID: 25666808 Free PMC article.
-
Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5454-63. doi: 10.1073/pnas.1607436113. Epub 2016 Aug 29. Proc Natl Acad Sci U S A. 2016. PMID: 27573839 Free PMC article.
-
Effects of anchor structure and glycosylation of Fcγ receptor III on ligand binding affinity.Mol Biol Cell. 2016 Nov 7;27(22):3449-3458. doi: 10.1091/mbc.E16-06-0470. Epub 2016 Aug 31. Mol Biol Cell. 2016. PMID: 27582391 Free PMC article.
-
Strategy of lipid recognition by invariant natural killer T cells: 'one for all and all for one'.Immunology. 2012 Jul;136(3):273-82. doi: 10.1111/j.1365-2567.2012.03580.x. Immunology. 2012. PMID: 22671023 Free PMC article. Review.
-
Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3ε cytoplasmic domain.J Exp Med. 2012 Dec 17;209(13):2423-39. doi: 10.1084/jem.20120790. Epub 2012 Nov 19. J Exp Med. 2012. PMID: 23166358 Free PMC article.
References
-
- Davis MM, et al. T cells as a self-referential, sensory organ. Annu. Rev. Immunol. 2007;25:681–695. - PubMed
-
- Grakoui A, et al. The immunological synapse: a molecular machine controlling cell activation. Science. 1999;285:221–227. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. - PubMed
-
- Corr M, et al. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994;265:946–949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

