Tumor Necrosis Factor-α Suppresses Activation of Sustained Potassium Currents in Rat Small Diameter Sensory Neurons
- PMID: 20165558
- PMCID: PMC2822358
- DOI: 10.2174/1876386300801010001
Tumor Necrosis Factor-α Suppresses Activation of Sustained Potassium Currents in Rat Small Diameter Sensory Neurons
Abstract
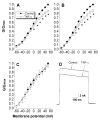
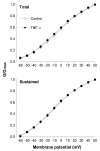
Tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, produces pain and hyperalgesia by activating and/or sensitizing nociceptive sensory neurons. In the present study, using whole-cell patch clamp techniques, the regulation of potassium currents by TNF-α was examined in acutely dissociated small dorsal root ganglion neurons. We found that acute application of TNF-α inhibited, in a dose-dependent manner, the non-inactivating sustained potassium current without changing the rapidly inactivating transient current or the kinetics of steady-state inactivation. The effects of TNF-α on potassium currents were similar to that of prostaglandin E2 as reported previously and also demonstrated in the current study. Furthermore, indomethacin, a potent inhibitor for both cyclo-oxygenase (COX) -1 and COX-2, completely blocked the effect of TNF-α on potassium currents. These results suggest that TNF-α may sensitize or activate sensory neurons by suppressing the sustained potassium current in nociceptive DRG neurons, possibly via stimulating the synthesis/release of endogenous prostaglandins.
Figures





References
-
- Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. - PubMed
-
- Budde T, Mager R, Pape HC. Different Types of Potassium Outward Current in Relay Neurons Acutely Isolated from the Rat Lateral Geniculate Nucleus. Eur J Neurosci. 1992;4:708–722. - PubMed
-
- Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJ. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum Reprod. 1995;10:2773–2780. - PubMed
-
- Creange A, Barlovatz-Meimon G, Gherardi RK. Cytokines and peripheral nerve disorders. Eur Cytokine Netw. 1997;8:145–151. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
