Targeting p53 for Novel Anticancer Therapy
- PMID: 20165689
- PMCID: PMC2822448
- DOI: 10.1593/tlo.09250
Targeting p53 for Novel Anticancer Therapy
Abstract
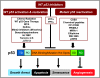
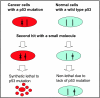
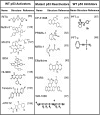
Carcinogenesis is a multistage process, involving oncogene activation and tumor suppressor gene inactivation as well as complex interactions between tumor and host tissues, leading ultimately to an aggressive metastatic phenotype. Among many genetic lesions, mutational inactivation of p53 tumor suppressor, the "guardian of the genome," is the most frequent event found in 50% of human cancers. p53 plays a critical role in tumor suppression mainly by inducing growth arrest, apoptosis, and senescence, as well as by blocking angiogenesis. In addition, p53 generally confers the cancer cell sensitivity to chemoradiation. Thus, p53 becomes the most appealing target for mechanism-driven anticancer drug discovery. This review will focus on the approaches currently undertaken to target p53 and its regulators with an overall goal either to activate p53 in cancer cells for killing or to inactivate p53 temporarily in normal cells for chemoradiation protection. The compounds that activate wild type (wt) p53 would have an application for the treatment of wt p53-containing human cancer. Likewise, the compounds that change p53 conformation from mutant to wt p53 (p53 reactivation) or that kill the cancer cells with mutant p53 using a synthetic lethal mechanism can be used to selectively treat human cancer harboring a mutant p53. The inhibitors of wt p53 can be used on a temporary basis to reduce the normal cell toxicity derived from p53 activation. Thus, successful development of these three classes of p53 modulators, to be used alone or in combination with chemoradiation, will revolutionize current anticancer therapies and benefit cancer patients.
Figures
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Linzer DI, Levine AJ. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17(1):43–52. - PubMed
-
- Lane DP, Crawford LV. Tantigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278(5701):261–263. - PubMed
-
- Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, van Tuinen P, Ledbetter DH, Barker DF, Nakamura Y, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244(4901):217–221. - PubMed
-
- Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57(7):1083–1093. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
