Evidence for DNA damage checkpoint activation in barrett esophagus
- PMID: 20165693
- PMCID: PMC2822452
- DOI: 10.1593/tlo.09187
Evidence for DNA damage checkpoint activation in barrett esophagus
Abstract
Barrett esophagus is an epithelial metaplasia that predisposes to adenocarcinoma. Better markers of cancer risk are urgently needed to identify those patients who are likely to benefit most from emerging methods of endoscopic ablation. Disease progression is associated with genomic DNA changes (segmental gains, losses, or loss of heterozygosity). Although these changes are not easily assayed directly, we hypothesized that the underlying DNA damage should activate a DNA damage response (DDR), detectable by immunohistochemical (IHC) assays of checkpoint proteins and the resulting replicative phase cell cycle delays. Surgical specimens and endoscopic biopsies (N = 28) were subjected to IHC for the cell cycle markers cyclin A and phosphorylated histone H3 (P-H3), the DDR markers gammaH2AX and phosphorylated ATM/ATR substrates (P-ATM/ATRsub), and the DNA damage-responsive tumor suppressors p16 and p53. Correlations were made with histologic diagnoses. The fractions of cells that stained for cyclin A, P-H3, and gammaH2AX increased in parallel in dysplastic tissue, consistent with checkpoint-mediated cell cycle delays. Foci of nuclear gammaH2AX and P-ATM/ATRsub were demonstrated by standard and confocal immunofluorescence. Staining for p16 was more prevalent in early-stage disease with lower staining for gammaH2AX and P-H3. Staining for p53 was moderately increased in some early-stage disease and strongly increased in some advanced disease, consistent with checkpoint-mediated induction and mutational inactivation of p53, respectively. We suggest that IHC for DDR-associated markers may help stratify risk of disease progression in Barrett.
Figures
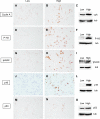
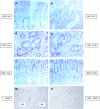
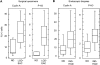
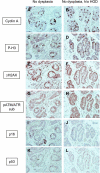

Similar articles
-
Expression of the cyclin-dependent kinase inhibitor p21(WAF1/CIP1) and p53 tumor suppressor in dysplastic progression and adenocarcinoma in Barrett esophagus.Cancer. 1999 Sep 1;86(5):756-63. doi: 10.1002/(sici)1097-0142(19990901)86:5<756::aid-cncr9>3.0.co;2-x. Cancer. 1999. PMID: 10463972
-
Activation of DNA damage response signaling in mouse embryonic stem cells.Cell Cycle. 2008 Sep 15;7(18):2922-8. doi: 10.4161/cc.7.18.6699. Epub 2008 Sep 30. Cell Cycle. 2008. PMID: 18787397
-
Nuclear translocation of Cyclin B1 marks the restriction point for terminal cell cycle exit in G2 phase.Cell Cycle. 2014;13(17):2733-43. doi: 10.4161/15384101.2015.945831. Cell Cycle. 2014. PMID: 25486360 Free PMC article.
-
Epidemiology and molecular biology of Barrett esophagus.Semin Thorac Cardiovasc Surg. 2005 Winter;17(4):284-91. doi: 10.1053/j.semtcvs.2005.09.005. Semin Thorac Cardiovasc Surg. 2005. PMID: 16428034 Review.
-
DNA damage checkpoint kinases in cancer.Expert Rev Mol Med. 2020 Jun 8;22:e2. doi: 10.1017/erm.2020.3. Expert Rev Mol Med. 2020. PMID: 32508294 Review.
Cited by
-
The emerging role of epigenetic modifiers in repair of DNA damage associated with chronic inflammatory diseases.Mutat Res Rev Mutat Res. 2019 Apr-Jun;780:69-81. doi: 10.1016/j.mrrev.2017.09.005. Epub 2017 Sep 28. Mutat Res Rev Mutat Res. 2019. PMID: 31395351 Free PMC article. Review.
-
Isoforms of RNF128 Regulate the Stability of Mutant P53 in Barrett's Esophageal Cells.Gastroenterology. 2020 Feb;158(3):583-597.e1. doi: 10.1053/j.gastro.2019.10.040. Epub 2019 Nov 9. Gastroenterology. 2020. PMID: 31715145 Free PMC article.
-
Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma.JAMA. 2011 Jul 27;306(4):410-9. doi: 10.1001/jama.2011.1029. JAMA. 2011. PMID: 21791690 Free PMC article.
-
From genetics to signaling pathways: molecular pathogenesis of esophageal adenocarcinoma.Biochim Biophys Acta Rev Cancer. 2019 Aug;1872(1):37-48. doi: 10.1016/j.bbcan.2019.05.003. Epub 2019 May 30. Biochim Biophys Acta Rev Cancer. 2019. PMID: 31152823 Free PMC article. Review.
-
Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation.Oncotarget. 2015 Oct 20;6(32):33438-55. doi: 10.18632/oncotarget.5586. Oncotarget. 2015. PMID: 26378019 Free PMC article.
References
-
- Winters C, Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, III, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS, et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. - PubMed
-
- Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. - PubMed
-
- Dulai GS, Shekelle PG, Jensen DM, Spiegel BM, Chen J, Oh D, Kahn KL. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett's cohort. Am J Gastroenterol. 2005;100:775–783. - PubMed
-
- Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. - PubMed
-
- Spechler SJ. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol. 2005;100:927–935. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
