Inhibition of thromboxane synthase activity improves glioblastoma response to alkylation chemotherapy
- PMID: 20165694
- PMCID: PMC2822453
- DOI: 10.1593/tlo.09238
Inhibition of thromboxane synthase activity improves glioblastoma response to alkylation chemotherapy
Abstract
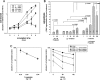
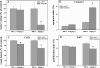
Thromboxane synthase (TXSA), an enzyme of the arachidonic acid metabolism, is upregulated in human glial tumors and is involved in glioma progression. Here, we analyzed the in vitro and in vivo effects of pharmacological inhibition of TXSA activity on human glioblastoma cells. Furegrelate, a specific inhibitor of TXSA, significantly inhibited tumor growth in an orthotopic glioblastoma model by inducing proapoptotic, antiproliferative, and antiangiogenic effects. Inhibition of TXSA induced a proapoptotic disposition of glioma cells and increased the sensitivity to the chemotherapeutic agent 1,3-bis(2-chloroethyl)-1-nitrosourea, significantly prolonging the survival time of intracerebral glioma-bearing mice. Our data demonstrate that the targeted inhibition of TXSA activity improves the efficiency of conventional alkylation chemotherapy in vivo. Our study supports the role of TXSA activity for the progression of malignant glioma and the potential utility of its therapeutic modulation for glioma treatment.
Figures


Similar articles
-
Thromboxane synthase inhibitors induce apoptosis in migration-arrested glioma cells.Neurosurgery. 2002 Feb;50(2):343-54. doi: 10.1097/00006123-200202000-00021. Neurosurgery. 2002. PMID: 11844270
-
Inhibition of invasion-associated thromboxane synthase sensitizes experimental gliomas to gamma-radiation.J Neurooncol. 2009 Feb;91(3):241-9. doi: 10.1007/s11060-008-9708-0. Epub 2008 Sep 30. J Neurooncol. 2009. PMID: 18825315
-
CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas.Clin Cancer Res. 2006 Nov 15;12(22):6765-71. doi: 10.1158/1078-0432.CCR-06-1372. Clin Cancer Res. 2006. PMID: 17121897
-
Inhibition of the arachidonic acid metabolism blocks endothelial cell migration and induces apoptosis.Acta Neurochir (Wien). 2004 May;146(5):483-94. doi: 10.1007/s00701-004-0238-z. Epub 2004 Apr 13. Acta Neurochir (Wien). 2004. PMID: 15118886
-
NO-mediated chemoresistance in C6 glioma cells.Ann N Y Acad Sci. 2002 May;962:8-17. doi: 10.1111/j.1749-6632.2002.tb04052.x. Ann N Y Acad Sci. 2002. PMID: 12076959 Review.
Cited by
-
The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection.Molecules. 2022 Sep 22;27(19):6234. doi: 10.3390/molecules27196234. Molecules. 2022. PMID: 36234768 Free PMC article. Review.
-
The thromboxane synthase and receptor signaling pathway in cancer: an emerging paradigm in cancer progression and metastasis.Cancer Metastasis Rev. 2011 Dec;30(3-4):397-408. doi: 10.1007/s10555-011-9297-9. Cancer Metastasis Rev. 2011. PMID: 22037941 Free PMC article. Review.
-
A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care.Oncotarget. 2013 Apr;4(4):502-30. doi: 10.18632/oncotarget.969. Oncotarget. 2013. PMID: 23594434 Free PMC article. Review.
-
Cyclooxygenases and lipoxygenases in cancer.Cancer Metastasis Rev. 2011 Dec;30(3-4):277-94. doi: 10.1007/s10555-011-9310-3. Cancer Metastasis Rev. 2011. PMID: 22002716 Free PMC article. Review.
-
Perifosine, a Bioavailable Alkylphospholipid Akt Inhibitor, Exhibits Antitumor Activity in Murine Models of Cancer Brain Metastasis Through Favorable Tumor Exposure.Front Oncol. 2021 Nov 4;11:754365. doi: 10.3389/fonc.2021.754365. eCollection 2021. Front Oncol. 2021. PMID: 34804943 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. - PubMed
-
- Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, Hall WA, Hynynen K, Senter PD, Peereboom DM, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–2305. - PubMed
-
- Westphal M, Lamszus K. Other experimental therapies for glioma. Recent Results Cancer Res. 2009;171:155–164. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
