Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro
- PMID: 20165896
- PMCID: PMC2862922
- DOI: 10.1007/s10162-009-0206-7
Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro
Abstract
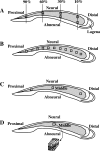
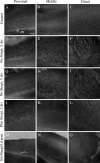
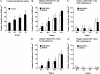
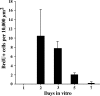
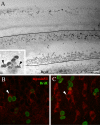
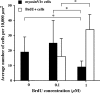
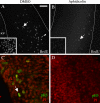
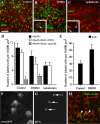
In chickens, nonsensory supporting cells divide and regenerate auditory hair cells after injury. Anatomical evidence suggests that supporting cells can also transdifferentiate into hair cells without dividing. In this study, we characterized an organ culture model to study auditory hair cell regeneration, and we used these cultures to test if direct transdifferentiation alone can lead to significant hair cell regeneration. Control cultures (organs from posthatch chickens maintained without streptomycin) showed complete hair cell loss in the proximal (high-frequency) region by 5 days. In contrast, a 2-day treatment with streptomycin induced loss of hair cells from all regions by 3 days. Hair cell regeneration proceeded in culture, with the time course of supporting cell division and hair cell differentiation generally resembling in vivo patterns. The degree of supporting cell division depended upon the presence of streptomycin, the epithelial region, the type of culture media, and serum concentration. On average, 87% of the regenerated hair cells lacked the cell division marker BrdU despite its continuous presence, suggesting that most hair cells were regenerated via direct transdifferentiation. Addition of the DNA polymerase inhibitor aphidicolin to culture media prevented supporting cell division, but numerous hair cells were regenerated nonetheless. These hair cells showed signs of functional maturation, including stereociliary bundles and rapid uptake of FM1-43. These observations demonstrate that direct transdifferentiation is a significant mechanism of hair cell regeneration in the chicken auditory after streptomycin damage in vitro.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

