Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): a pilot study
- PMID: 20165976
- PMCID: PMC2865176
- DOI: 10.1007/s10620-010-1146-0
Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): a pilot study
Abstract
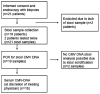
Background: A severe flare of colitis in patients with IBD treated with immunosuppressive therapy may be complicated by an underlying CMV infection. The aim of this pilot study was to investigate the diagnostic efficacy of quantitative polymerase chain reaction (PCR) to detect CMV DNA in stool samples of IBD patients.
Methods: Twenty-one patients with a severe flare of IBD, incompletely responding or refractory to either steroids or immunosuppressive agents, were included in the study. Nineteen patients completed the study according to the protocol undergoing an endoscopy with biopsies and collection of stool samples. Biopsy and stool samples were qualitatively and quantitatively analyzed for CMV DNA using real-time PCR.
Results: Thirty-two percent (6/19) of the patients had detectable CMV DNA in colonic biopsies and in five (83%) of those patients CMV DNA was detected in the stool. Thirteen patients had negative findings for CMV DNA in biopsy and stool samples. The sensitivity, specificity, and accuracy of the PCR-based stool test for detection of CMV DNA compared to PCR-based detection of CMV in mucosal biopsies were 83, 93, and 90%, respectively.
Conclusions: The pilot study suggests a high accuracy of this non-invasive testing method to detect CMV DNA in stool samples as compared to mucosal biopsies. This approach may offer a non-endoscopic testing modality for underlying CMV infection in patients with a severe flare of IBD, which could also be applied more broadly to determine the prevalence of CMV infections in patients with IBD.
References
-
- Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005;50:609–616. - PubMed
-
- Buckner FS, Pomeroy C. Cytomegalovirus disease of the gastrointestinal tract in patients without aids. Clin Infect Dis. 1993;17:644–656. - PubMed
-
- Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365–373. - PubMed
-
- Schulenburg A, Turetschek K, Wrba F, et al. Early and late gastrointestinal complications after myeloablative and non-myeloablative allogeneic stem cell transplantation. Ann Hematol. 2004;83:101–106. - PubMed