Chronic inflammatory responses to microgel-based implant coatings
- PMID: 20166218
- PMCID: PMC2875296
- DOI: 10.1002/jbm.a.32669
Chronic inflammatory responses to microgel-based implant coatings
Abstract
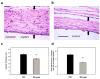
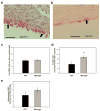
Inflammatory responses to implanted biomedical devices elicit a foreign body fibrotic reaction that limits device integration and performance in various biomedical applications. We examined chronic inflammatory responses to microgel conformal coatings consisting of thin films of poly(N-isopropylacrylamide) hydrogel microparticles cross-linked with poly(ethylene glycol) diacrylate deposited on poly(ethylene terephthalate) (PET). Unmodified and microgel-coated PET disks were implanted subcutaneously in rats for 4 weeks and explants were analyzed by histology and immunohistochemistry. Microgel coatings reduced chronic inflammation and resulted in a more mature/organized fibrous capsule. Microgel-coated samples exhibited 22% thinner fibrous capsules that contained 40% fewer cells compared to unmodified PET disks. Furthermore, microgel-coated samples contained significantly higher levels of macrophages (80%) than unmodified PET controls. These results demonstrate that microgel coatings reduce chronic inflammation to implanted biomaterials. (c) 2010 Wiley Periodicals, Inc. J Biomed Mater Res, 2010.
Figures
Similar articles
-
Reduced acute inflammatory responses to microgel conformal coatings.Biomaterials. 2008 Dec;29(35):4605-15. doi: 10.1016/j.biomaterials.2008.08.015. Epub 2008 Sep 19. Biomaterials. 2008. PMID: 18804859 Free PMC article.
-
Reducing the foreign body response on human cochlear implants and their materials in vivo with photografted zwitterionic hydrogel coatings.Acta Biomater. 2023 Aug;166:212-223. doi: 10.1016/j.actbio.2023.05.011. Epub 2023 May 13. Acta Biomater. 2023. PMID: 37187301 Free PMC article.
-
Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels.Acta Biomater. 2019 Dec;100:105-117. doi: 10.1016/j.actbio.2019.09.043. Epub 2019 Sep 27. Acta Biomater. 2019. PMID: 31568879 Free PMC article.
-
Silane coatings of metallic biomaterials for biomedical implants: A preliminary review.J Biomed Mater Res B Appl Biomater. 2018 Nov;106(8):2901-2918. doi: 10.1002/jbm.b.34151. Epub 2018 Aug 9. J Biomed Mater Res B Appl Biomater. 2018. PMID: 30091505 Review.
-
Macrophage responses to implants: prospects for personalized medicine.J Leukoc Biol. 2015 Dec;98(6):953-62. doi: 10.1189/jlb.5VMR0415-166R. Epub 2015 Jul 13. J Leukoc Biol. 2015. PMID: 26168797 Review.
Cited by
-
Ultralow protein adsorbing coatings from clickable PEG nanogel solutions: benefits of attachment under salt-induced phase separation conditions and comparison with PEG/albumin nanogel coatings.Langmuir. 2013 Mar 26;29(12):4128-39. doi: 10.1021/la3051115. Epub 2013 Mar 11. Langmuir. 2013. PMID: 23441808 Free PMC article.
-
Methods for Generating Hydrogel Particles for Protein Delivery.Ann Biomed Eng. 2016 Jun;44(6):1946-58. doi: 10.1007/s10439-016-1637-z. Epub 2016 May 9. Ann Biomed Eng. 2016. PMID: 27160672 Free PMC article. Review.
-
Modulation of the foreign body reaction for implants in the subcutaneous space: microdialysis probes as localized drug delivery/sampling devices.J Diabetes Sci Technol. 2011 May 1;5(3):619-31. doi: 10.1177/193229681100500316. J Diabetes Sci Technol. 2011. PMID: 21722577 Free PMC article.
-
Particle Stiffness and Surface Topography Determine Macrophage-Mediated Removal of Surface Adsorbed Particles.Adv Healthc Mater. 2021 Mar;10(6):e2001667. doi: 10.1002/adhm.202001667. Epub 2021 Jan 12. Adv Healthc Mater. 2021. PMID: 33434386 Free PMC article.
-
Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings.Bioact Mater. 2021 Apr 3;6(10):3634-3657. doi: 10.1016/j.bioactmat.2021.03.004. eCollection 2021 Oct. Bioact Mater. 2021. PMID: 33898869 Free PMC article. Review.
References
-
- Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110.
-
- Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25(26):5681–703. - PubMed
-
- Kottke-Marchant K, Anderson JM, Umemura Y, Marchant RE. Effect of albumin coating on the in vitro blood compatibility of Dacron arterial prostheses. Biomaterials. 1989;10(3):147–55. - PubMed
-
- Zhao Q, Topham N, Anderson JM, Hiltner A, Lodoen G, Payet CR. Foreign-body giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res. 1991;25(2):177–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

