Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula
- PMID: 20166701
- PMCID: PMC2846190
- DOI: 10.1021/np900614n
Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula
Abstract
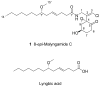
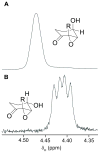
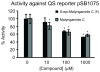
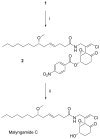
A new stereoisomer of malyngamide C, 8-epi-malyngamide C (1), and the known compound lyngbic acid [(4E,7S)-7-methoxytetradec-4-enoic acid] were isolated from a sample of Lyngbya majuscula collected near Bush Key, Dry Tortugas, Florida. The structure of 1 was determined by NMR and MS experiments. The absolute configuration of 1 was determined by selective Mitsunobu inversion of C-8 to give malyngamide C, as determined by NMR, MS, and comparison of specific rotation. Both 1 and malyngamide C were found to be cytotoxic to HT29 colon cancer cells (IC(50) 15.4 and 5.2 microM, respectively) and to inhibit bacterial quorum sensing in a reporter gene assay.
Figures
Similar articles
-
Two cytotoxic stereoisomers of malyngamide C, 8-epi-malyngamide C and 8-O-acetyl-8-epi-malyngamide C, from the marine cyanobacterium Lyngbya majuscula.Phytochemistry. 2010 Oct;71(14-15):1729-35. doi: 10.1016/j.phytochem.2010.07.001. Epub 2010 Aug 9. Phytochemistry. 2010. PMID: 20701935 Free PMC article.
-
Malyngamide H, an ichthyotoxic amide possessing a new carbon skeleton from the Caribbean cyanobacterium Lyngbya majuscula.J Nat Prod. 1995 May;58(5):764-8. doi: 10.1021/np50119a019. J Nat Prod. 1995. PMID: 7623050
-
Three new malyngamides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula.J Nat Prod. 2003 Jan;66(1):132-5. doi: 10.1021/np0204186. J Nat Prod. 2003. PMID: 12542362
-
Malyngamide 3 and cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from Cocos Lagoon, Guam.J Nat Prod. 2011 Apr 25;74(4):871-6. doi: 10.1021/np1008015. Epub 2011 Feb 22. J Nat Prod. 2011. PMID: 21341718 Free PMC article.
-
A new biologically active malyngamide from a New Zealand collection of the sea hare Bursatella leachii.J Nat Prod. 2002 Apr;65(4):630-1. doi: 10.1021/np010511e. J Nat Prod. 2002. PMID: 11975522
Cited by
-
Studies toward the Synthesis of Smenamide A, an Antiproliferative Metabolite from Smenospongia aurea: Total Synthesis of ent-Smenamide A and 16-epi-Smenamide A.ACS Omega. 2017 Apr 30;2(4):1477-1488. doi: 10.1021/acsomega.7b00095. Epub 2017 Apr 17. ACS Omega. 2017. PMID: 30023636 Free PMC article.
-
Inhibition of marine biofouling by bacterial quorum sensing inhibitors.Biofouling. 2011 Sep;27(8):893-905. doi: 10.1080/08927014.2011.609616. Biofouling. 2011. PMID: 21882898 Free PMC article.
-
Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer.Mar Drugs. 2015 Sep 30;13(10):6152-209. doi: 10.3390/md13106152. Mar Drugs. 2015. PMID: 26437418 Free PMC article. Review.
-
Modular strategies for structure and function employed by marine cyanobacteria: characterization and synthesis of pitinoic acids.Org Lett. 2013 Aug 16;15(16):4050-3. doi: 10.1021/ol401396u. Epub 2013 Aug 5. Org Lett. 2013. PMID: 23915229 Free PMC article.
-
Credneramides A and B: neuromodulatory phenethylamine and isopentylamine derivatives of a vinyl chloride-containing fatty acid from cf. Trichodesmium sp. nov.J Nat Prod. 2012 Jan 27;75(1):60-6. doi: 10.1021/np200611f. Epub 2011 Dec 12. J Nat Prod. 2012. PMID: 22148360 Free PMC article.
References
-
- Ainslie RD, Barchi JJ, Kuniyoshi M, Moore RE, Mynderse JS. J Org Chem. 1985;50:2859–2862.
-
- Cardellina JH, Dalietos D, Marner FJ, Mynderse JS, Moore RE. Phytochemistry. 1978;17:2091–2095.
-
- Cardellina JH, Marner FJ, Moore RE. J Am Chem Soc. 1979;101:240–242.
-
- Moore RE. In: Marine Natural Products: Chemical and Biological Perspectives. Scheuer PJ, editor. IV. Academic Press; New York: 1981. pp. 1–52.
-
- Mynderse JS, Moore RE. J Org Chem. 1978;43:4359–4363.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

