Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity
- PMID: 20166790
- PMCID: PMC2835389
- DOI: 10.1089/cap.2009.0059
Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity
Abstract
Objective: Guanfacine has been shown to reduce hyperactive behaviors in children with attention-deficit/hyperactivity disorder (ADHD) and possibly in children with pervasive developmental disorder (PDD) and hyperactivity. The aim of this exploratory study was to examine whether gene variants encoding the multidrug resistance protein (MDR1 or ABCB1) , a drug transporter at the blood-brain barrier, are associated with variability in the efficacy of guanfacine in children with PDD and hyperactivity.
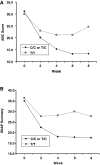
Methods: Children with PDD who participated in an 8-week open-label trial of guanfacine were genotyped for the C3435T single-nucleotide polymorphism (SNP) variant of the MDR1 gene, a variant reported to alter function of the transporter. The decrease from baseline to 8 weeks in parent-rated Aberrant Behavior Checklist (ABC) hyperactivity and Swanson, Nolan, and Pelham (SNAP) scores were analyzed by MDR1 genotype. Response was compared between subjects homozygous for the minor allele T of the C34535T MDR1 variant (T/T) versus other genotypes (C/T and C/C).
Results: Disruptive behavior decreased during guanfacine treatment as assessed by several end points in the 25 enrolled children (23 boys and 2 girls). Genotype data were available from 22 children. Subjects with either C/T or C/C (n = 16) genotypes showed a three-fold greater improvement than T/T MDR1 C3435T genotype (n = 6) (mean decrease of 15.1 +/- 12.6, or 50.7% from baseline, versus 4.5 +/- 5.1, or 15.6% from baseline) in parent-rated ABC Hyperactivity scores over 8 weeks (p = 0.03). Parent-rated ADHD SNAP scores also differed by genotype (p = 0.05).
Conclusions: Gene variants in MDR1 may influence guanfacine response on hyperactive-impulsive behaviors via altered membrane transport. If replicated in larger samples, additional studies would be important to clarify the mechanisms underlying this effect and to determine its clinical significance. 2.
Figures
Similar articles
-
A prospective open trial of guanfacine in children with pervasive developmental disorders.J Child Adolesc Psychopharmacol. 2006 Oct;16(5):589-98. doi: 10.1089/cap.2006.16.589. J Child Adolesc Psychopharmacol. 2006. PMID: 17069547
-
Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases.J Child Adolesc Psychopharmacol. 2004 Summer;14(2):233-41. doi: 10.1089/1044546041649084. J Child Adolesc Psychopharmacol. 2004. PMID: 15319020
-
A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder.Am J Psychiatry. 2001 Jul;158(7):1067-74. doi: 10.1176/appi.ajp.158.7.1067. Am J Psychiatry. 2001. PMID: 11431228 Clinical Trial.
-
Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness.CNS Drugs. 2009;23 Suppl 1:43-9. doi: 10.2165/00023210-200923000-00006. CNS Drugs. 2009. PMID: 19621977 Review.
-
Guanfacine and guanfacine extended release: treatment for ADHD and related disorders.CNS Drug Rev. 2007 Winter;13(4):465-74. doi: 10.1111/j.1527-3458.2007.00026.x. CNS Drug Rev. 2007. PMID: 18078429 Free PMC article. Review.
Cited by
-
Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses.Neuron. 2012 Oct 4;76(1):223-39. doi: 10.1016/j.neuron.2012.08.038. Neuron. 2012. PMID: 23040817 Free PMC article. Review.
-
An in vitro evaluation of guanfacine as a substrate for P-glycoprotein.Neuropsychiatr Dis Treat. 2011;7:501-5. doi: 10.2147/NDT.S24153. Epub 2011 Aug 26. Neuropsychiatr Dis Treat. 2011. PMID: 21931492 Free PMC article.
-
Scientific rationale for the use of α2A-adrenoceptor agonists in treating neuroinflammatory cognitive disorders.Mol Psychiatry. 2023 Nov;28(11):4540-4552. doi: 10.1038/s41380-023-02057-4. Epub 2023 Apr 7. Mol Psychiatry. 2023. PMID: 37029295 Free PMC article. Review.
-
Targeting Prefrontal Cortical Systems for Drug Development: Potential Therapies for Cognitive Disorders.Annu Rev Pharmacol Toxicol. 2016;56:339-60. doi: 10.1146/annurev-pharmtox-010715-103617. Annu Rev Pharmacol Toxicol. 2016. PMID: 26738476 Free PMC article. Review.
-
Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions.J Clin Med. 2025 Jun 5;14(11):4000. doi: 10.3390/jcm14114000. J Clin Med. 2025. PMID: 40507763 Free PMC article. Review.
References
-
- Aman MG. Singh NN. Stewart AW. Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Deficiency. 1985;89:485–491. - PubMed
-
- Aman MG. Buican B. Arnold LE. Methylphenidate treatment in children with borderline IQ and mental retardation: Analysis of three aggregated studies. J Child Adoles Psychopharmacol. 2003;13:27–38. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. (DSM-IV).
-
- Biederman J. Melmed RD. Patel A. McBurnett K. Konow J. Lyne A. Scherer N. SPD503 Study Group: A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008a;121:e73–e84. - PubMed
-
- Biederman J. Melmed RD. Patel A. McBurnett K. Donahue J. Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008b;13:1047–1055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical