Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease
- PMID: 20166878
- PMCID: PMC2871072
- DOI: 10.3109/08916930903405891
Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease
Abstract
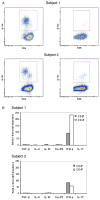
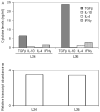
Kawasaki disease (KD) is a pediatric self-limited vasculitis characterized by immune-mediated destruction of the arterial wall and myocardium. Neither the trigger that incites the inflammation nor the switch that turns it off is known. To further our understanding of KD pathogenesis and the role of regulatory T-cells in modulating the inflammatory response, we studied circulating effector memory T-cells (CCR7- and IL-15+ T(em)) and central memory T-cells (CCR7+ and IL-15+ T(cm)) in six KD subjects. In two of the subjects, we cloned the remaining T-cell population by limiting dilution. TaqMan analysis of T(em) studied in two KD subjects suggested that T(em) are pro-inflammatory CD4+T-helper 1 cells and CD8+ cytotoxic T-cells. Following memory T-cells over time, we defined that circulating T(em) and T(cm) are detectable during the acute phase in some KD subjects before treatment with intravenous immunoglobulin. Both T(em) and T(cm) expand rapidly within 2 weeks of treatment. The circulating T(em) pool contracts, while T(cm) further proliferate in the convalescent phase. Following depletion of memory T-cells, numerous T-cell clones were derived from two acute KD subjects. The large majority of these T-cells displayed the functional phenotype of peripherally induced regulatory T-cells (T(reg)). These findings provide insight into the nature and kinetics of the adaptive immune response in KD.
Conflict of interest statement
Figures



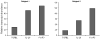
References
-
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364:533–544. - PubMed
-
- Burns JC. The riddle of Kawasaki disease. N Engl J Med. 2007;356:659–661. - PubMed
-
- Brugner D, Davila S, Breunis WB, Ng SB, Bonnard C, Ling L, Wright VJ, Thalamuthu A, Odam M, Shimizu C, Burns JC, Levin M, Kuijpers TW, Hibberd ML. A genomic wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
