Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown
- PMID: 20166921
- PMCID: PMC4120766
- DOI: 10.2174/092986710790980005
Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown
Abstract
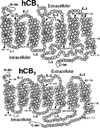
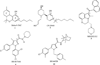
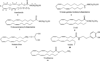
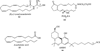
The cannabinoid CB1 and CB2 receptors are Class A G protein-coupled receptors (GPCRs). While many Class A GPCRs have endogenous ligands that are hydrophilic cations (e.g., the serotonin and dopamine receptors), the cannabinoid receptors have neutral, highly lipophilic ligands derived from the fatty acid, arachidonic acid. The most well-studied of these are N-arachidonoylethanolamine (anandamide, AEA) and sn-2-arachidonoylglycerol (2-AG). This review focuses on the experimental and computational studies that have been used to probe the nature of endocannabinoid interaction with the cannabinoid receptors. These studies include mutation, SAR and NMR studies, as well as, QSAR, docking and molecular dynamics simulations. Gaps in our knowledge are identified. The review begins more generally, however, by discussing the entire endocannabinoid system, of which the cannabinoid receptors are part. For in order to understand endocannabinoid action, one needs an appreciation for the environments for which these ligands have been designed and the conformational changes these ligands must undergo in order to act on the cannabinoid receptors.
Figures


References
-
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. - PubMed
-
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. - PubMed
-
- Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. - PubMed
-
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

