Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists
- PMID: 20166927
- PMCID: PMC3013229
- DOI: 10.2174/092986710790980050
Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists
Abstract
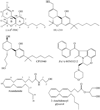
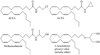
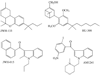
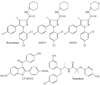
It is widely accepted that non-endogenous compounds that target CB(1) and/or CB(2) receptors possess therapeutic potential for the clinical management of an ever growing number of disorders. Just a few of these disorders are already treated with Delta(9)-tetrahydrocannabinol or nabilone, both CB(1)/CB(2) receptor agonists, and there is now considerable interest in expanding the clinical applications of such agonists and also in exploiting CB(2)-selective agonists, peripherally restricted CB(1)/CB(2) receptor agonists and CB(1)/CB(2) antagonists and inverse agonists as medicines. Already, numerous cannabinoid receptor ligands have been developed and their interactions with CB(1) and CB(2) receptors well characterized. This review describes what is currently known about the ability of such compounds to bind to, activate, inhibit or block non-CB(1), non- CB(2) G protein-coupled receptors such as GPR55, transmitter gated channels, ion channels and nuclear receptors in an orthosteric or allosteric manner. It begins with a brief description of how each of these ligands interacts with CB(1) and/or CB(2) receptors.
Figures
References
-
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. - PubMed
-
- Pertwee RG. Pharmacological actions of cannabinoids. In: Pertwee RG, editor. Cannabinoids. Handbook of Experimental Pharmacology. Vol. 168. Heidelberg: Springer-Verlag; 2005. pp. 1–51. - PubMed
-
- Pertwee RG. Cannabinoids and multiple sclerosis. Mol. Neurobiol. 2007;36:45–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

