Design and application of microfluidic systems for in vitro pharmacokinetic evaluation of drug candidates
- PMID: 20166997
- PMCID: PMC3206315
- DOI: 10.2174/138920009790820093
Design and application of microfluidic systems for in vitro pharmacokinetic evaluation of drug candidates
Abstract
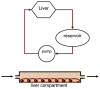
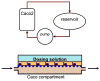
One of the fundamental challenges facing the development of new chemical entities within the pharmaceutical industry is the extrapolation of key in vivo parameters from in vitro cell culture assays and animal studies. Development of microscale devices and screening assays incorporating primary human cells can potentially provide better, faster and more efficient prediction of in vivo toxicity and clinical drug performance. With this goal in mind, large strides have been made in the area of microfluidics to provide in vitro surrogates that are designed to mimic the physiological architecture and dynamics. More recent advancements have been made in the development of in vitro analogues to physiologically-based pharmacokinetic (PBPK) models - a mathematical model that represents the body as interconnected compartments specific for a particular organ. In this review we highlight recent advancements in human hepatocyte microscale culture, and describe the next generation of integrated devices, whose potential allows for the high throughput assessment of drug metabolism, distribution and pharmacokinetics.
Figures
Similar articles
-
Organ-on-a-chip technology and microfluidic whole-body models for pharmacokinetic drug toxicity screening.Biotechnol J. 2013 Nov;8(11):1258-66. doi: 10.1002/biot.201300086. Epub 2013 Aug 23. Biotechnol J. 2013. PMID: 24038956 Review.
-
Hurel -- an in vivo-surrogate assay platform for cell-based studies.Altern Lab Anim. 2009 Sep;37 Suppl 1:11-8. doi: 10.1177/026119290903701S01. Altern Lab Anim. 2009. PMID: 19807199
-
Metabolic stability for drug discovery and development: pharmacokinetic and biochemical challenges.Clin Pharmacokinet. 2003;42(6):515-28. doi: 10.2165/00003088-200342060-00002. Clin Pharmacokinet. 2003. PMID: 12793837 Review.
-
Preclinical pharmacokinetics: an approach towards safer and efficacious drugs.Curr Drug Metab. 2006 Feb;7(2):165-82. doi: 10.2174/138920006775541552. Curr Drug Metab. 2006. PMID: 16472106 Review.
-
Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:37-64. doi: 10.1146/annurev-pharmtox-010716-104748. Annu Rev Pharmacol Toxicol. 2018. PMID: 29309256 Review.
Cited by
-
Comparison of the degradation behavior of PLGA scaffolds in micro-channel, shaking, and static conditions.Biomicrofluidics. 2018 May 18;12(3):034106. doi: 10.1063/1.5021394. eCollection 2018 May. Biomicrofluidics. 2018. PMID: 29861809 Free PMC article.
-
Microfluidics - The State-of-the-Art Technology for Pharmaceutical Application.Adv Pharm Bull. 2022 Aug;12(4):700-711. doi: 10.34172/apb.2022.073. Epub 2021 Sep 29. Adv Pharm Bull. 2022. PMID: 36415637 Free PMC article. Review.
-
Flow-perfusion bioreactor system for engineered breast cancer surrogates to be used in preclinical testing.J Tissue Eng Regen Med. 2017 Apr;11(4):1242-1250. doi: 10.1002/term.2026. Epub 2015 May 7. J Tissue Eng Regen Med. 2017. PMID: 25950420 Free PMC article.
-
The Combination of Electrochemistry and Microfluidic Technology in Drug Metabolism Studies.ChemistryOpen. 2022 Dec;11(12):e202200100. doi: 10.1002/open.202200100. Epub 2022 Sep 27. ChemistryOpen. 2022. PMID: 36166688 Free PMC article. Review.
-
Microfluidics-based in vivo mimetic systems for the study of cellular biology.Acc Chem Res. 2014 Apr 15;47(4):1165-73. doi: 10.1021/ar4002608. Epub 2014 Feb 20. Acc Chem Res. 2014. PMID: 24555566 Free PMC article.
References
-
- Chaturvedi PR, Decker CJ, Odinecs A. Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr Opin Chem Biol. 2001;5(4):452–463. - PubMed
-
- Korfmacher WA. Lead optimization strategies as part of a drug metabolism environment. Curr Opin Drug Discov Dev. 2003;6(4):481–485. - PubMed
-
- Rathore R, Jain JP, Srivastava A, Jachak SM, Kumar N. Simultaneous determination of hydrazinocurcumin and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal. 2008;46(2):374–380. - PubMed
-
- Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre-preclinical paradigm: compound optimization in early and late phase drug discovery. Curr Top Med Chem. 2001;1(5):353–366. - PubMed
-
- Tang L, Khan SU, Muhammad NA. Evaluation and selection of bio-relevant dissolution media for a poorly water-soluble new chemical entity. Pharm Dev Technol. 2001;6(4):531–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

