General and species-specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. riparia) grapevine species
- PMID: 20167053
- PMCID: PMC2831845
- DOI: 10.1186/1471-2164-11-117
General and species-specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. riparia) grapevine species
Abstract
Background: Downy mildew is a destructive grapevine disease caused by Plasmopara viticola (Berk. and Curt.) Berl. and de Toni, which can only be controlled by intensive fungicide treatments. Natural sources of resistance from wild grapevine (Vitis) species are used in conventional breeding approaches, but the signals and effectors involved in resistance in this important crop species are not well understood.
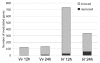
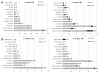
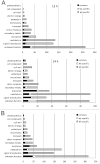
Results: Early transcriptional changes associated with P. viticola infection in susceptible V. vinifera and resistant V. riparia plants were analyzed using the Combimatrix microarray platform. Transcript levels were measured 12 and 24 h post-inoculation, reflecting the time points immediately preceding the onset of resistance in V. riparia, as determined by microscopic analysis. Our data indicate that resistance in V. riparia is induced after infection, and is not based on differences in basal gene expression between the two species. The strong and rapid transcriptional reprogramming involves the induction of pathogenesis-related proteins and enzymes required for the synthesis of phenylpropanoid-derived compounds, many of which are also induced, albeit to a lesser extent, in V. vinifera. More interestingly, resistance in V. riparia also involves the specific modulation of numerous transcripts encoding components of signal transduction cascades, hypersensitive reaction markers and genes involved in jasmonate biosynthesis. The limited transcriptional modulation in V. vinifera represents a weak attempted defense response rather than the activation of compatibility-specific pathways.
Conclusions: Several candidate resistance genes were identified that could be exploited in future biotechnological approaches to increase disease resistance in susceptible grapevine species. Measurements of jasmonic acid and methyl jasmonate in infected leaves suggest that this hormone may also be involved in V. riparia resistance to P. viticola.
Figures

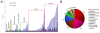
Similar articles
-
Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms.PLoS One. 2019 Sep 27;14(9):e0222854. doi: 10.1371/journal.pone.0222854. eCollection 2019. PLoS One. 2019. PMID: 31560730 Free PMC article.
-
Cultivar-specific kinetics of gene induction during downy mildew early infection in grapevine.Funct Integr Genomics. 2012 Jun;12(2):379-86. doi: 10.1007/s10142-012-0261-8. Epub 2012 Jan 14. Funct Integr Genomics. 2012. PMID: 22246600
-
Characterization of Vitis vinifera NPR1 homologs involved in the regulation of pathogenesis-related gene expression.BMC Plant Biol. 2009 May 11;9:54. doi: 10.1186/1471-2229-9-54. BMC Plant Biol. 2009. PMID: 19432948 Free PMC article.
-
Biotechnological Approaches: Gene Overexpression, Gene Silencing, and Genome Editing to Control Fungal and Oomycete Diseases in Grapevine.Int J Mol Sci. 2020 Aug 9;21(16):5701. doi: 10.3390/ijms21165701. Int J Mol Sci. 2020. PMID: 32784854 Free PMC article. Review.
-
The pathogenicity of Plasmopara viticola: a review of evolutionary dynamics, infection strategies and effector molecules.BMC Plant Biol. 2024 Apr 24;24(1):327. doi: 10.1186/s12870-024-05037-0. BMC Plant Biol. 2024. PMID: 38658826 Free PMC article. Review.
Cited by
-
Linking Jasmonic Acid to Grapevine Resistance against the Biotrophic Oomycete Plasmopara viticola.Front Plant Sci. 2016 Apr 28;7:565. doi: 10.3389/fpls.2016.00565. eCollection 2016. Front Plant Sci. 2016. PMID: 27200038 Free PMC article.
-
Current Knowledge and Computational Techniques for Grapevine Meta-Omics Analysis.Front Plant Sci. 2018 Jan 9;8:2241. doi: 10.3389/fpls.2017.02241. eCollection 2017. Front Plant Sci. 2018. PMID: 29375610 Free PMC article. Review.
-
Elevated atmospheric CO2 concentrations alter grapevine (Vitis vinifera) systemic transcriptional response to European grapevine moth (Lobesia botrana) herbivory.Sci Rep. 2019 Feb 28;9(1):2995. doi: 10.1038/s41598-019-39979-5. Sci Rep. 2019. PMID: 30816321 Free PMC article.
-
The Grapevine E3 Ubiquitin Ligase VriATL156 Confers Resistance against the Downy Mildew Pathogen Plasmopara viticola.Int J Mol Sci. 2021 Jan 19;22(2):940. doi: 10.3390/ijms22020940. Int J Mol Sci. 2021. PMID: 33477914 Free PMC article.
-
Plasmopara viticola Effector PvRXLR10 Targets a Host Phospholipase VvipPLA-IIδ2 to Suppress Plant Immunity in Grapevine.Mol Plant Pathol. 2025 May;26(5):e70095. doi: 10.1111/mpp.70095. Mol Plant Pathol. 2025. PMID: 40375562 Free PMC article.
References
-
- Grenville-Briggs LJ, van West P. The biotrophic stages of oomycete-plant interactions. Adv Appl Microbiol. 2005;57:217–243. full_text. - PubMed
-
- Grando MS, Bellin D, Edwards KJ, Pozzi C, Stefanini M, Velasco R. Molecular linkage maps of Vitis vinifera L. and Vitis riparia Mchx. Theor Appl Genet. 2003;106:1213–1224. - PubMed
-
- Merdinoglu D, Wiedeman-Merdinoglu S, Coste P, Dumas V, Haetty S, Butterlin G, Greif C. Genetic analysis of downy mildew resistance derived from Muscadinia rotundifolia. VIII International Conference on Grape Genetics and Breeding; Kecskemet, Hungary. 2003. pp. 451–456.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

