Anti-tetherin activities in Vpu-expressing primate lentiviruses
- PMID: 20167081
- PMCID: PMC2831821
- DOI: 10.1186/1742-4690-7-13
Anti-tetherin activities in Vpu-expressing primate lentiviruses
Abstract
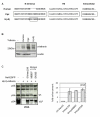
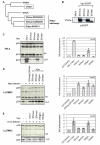
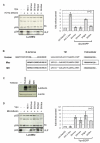
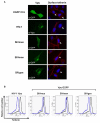
Background: The anti-viral activity of the cellular restriction factor, BST-2/tetherin, was first observed as an ability to block the release of Vpu-minus HIV-1 from the surface of infected cells. However, tetherin restriction is also counteracted by primate lentiviruses that do not express a Vpu protein, where anti-tetherin functions are provided by either the Env protein (HIV-2, SIVtan) or the Nef protein (SIVsm/mac and SIVagm). Within the primate lentiviruses, Vpu is also present in the genomes of SIVcpz and certain SIVsyk viruses. We asked whether, in these viruses, anti-tetherin activity was always a property of Vpu, or if it had selectively evolved in HIV-1 to perform this function.
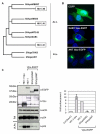
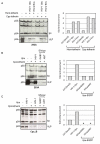
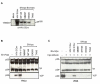
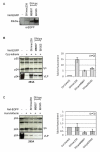
Results: We found that despite the close relatedness of HIV-1 and SIVcpz, the chimpanzee viruses use Nef instead of Vpu to counteract tetherin. Furthermore, SIVcpz Nef proteins had activity against chimpanzee but not human tetherin. This specificity mapped to a short sequence that is present in the cytoplasmic tail of primate but not human tetherins, and this also accounts for the specificity of SIVsm/mac Nef for primate but not human tetherins. In contrast, Vpu proteins from four diverse members of the SIVsyk lineage all displayed an anti-tetherin activity that was active against macaque tetherin. Interestingly, Vpu from a SIVgsn isolate was also found to have activity against human tetherin.
Conclusions: Primate lentiviruses show a high degree of flexibility in their use of anti-tetherin factors, indicating a strong selective pressure to counteract tetherin restriction. The identification of an activity against human tetherin in SIVgsn Vpu suggests that the presence of Vpu in the ancestral SIVmus/mon/gsn virus believed to have contributed the 3' half of the HIV-1 genome may have played a role in the evolution of viruses that could counteract human tetherin and infect humans.
Figures
Similar articles
-
Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses.J Virol. 2010 Jul;84(14):7124-34. doi: 10.1128/JVI.00468-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444900 Free PMC article.
-
Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2.PLoS Pathog. 2009 May;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436700 Free PMC article.
-
Vpu of a Simian Immunodeficiency Virus Isolated from Greater Spot-Nosed Monkey Antagonizes Human BST-2 via Two AxxxxxxxW Motifs.J Virol. 2020 Jan 6;94(2):e01669-19. doi: 10.1128/JVI.01669-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666374 Free PMC article.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
-
BST-2/tetherin: a new component of the innate immune response to enveloped viruses.Trends Microbiol. 2010 Sep;18(9):388-96. doi: 10.1016/j.tim.2010.06.010. Epub 2010 Aug 3. Trends Microbiol. 2010. PMID: 20688520 Free PMC article. Review.
Cited by
-
Ex vivo gene therapy for HIV-1 treatment.Hum Mol Genet. 2011 Apr 15;20(R1):R100-7. doi: 10.1093/hmg/ddr160. Epub 2011 Apr 19. Hum Mol Genet. 2011. PMID: 21505069 Free PMC article. Review.
-
Counteraction of the multifunctional restriction factor tetherin.Front Microbiol. 2014 Apr 10;5:163. doi: 10.3389/fmicb.2014.00163. eCollection 2014. Front Microbiol. 2014. PMID: 24782851 Free PMC article. Review.
-
Host restriction factors in retroviral infection: promises in virus-host interaction.Retrovirology. 2012 Dec 20;9:112. doi: 10.1186/1742-4690-9-112. Retrovirology. 2012. PMID: 23254112 Free PMC article. Review.
-
Breaking Barriers to an AIDS Model with Macaque-Tropic HIV-1 Derivatives.Biology (Basel). 2012 May 12;1(2):134-64. doi: 10.3390/biology1020134. Biology (Basel). 2012. PMID: 23336082 Free PMC article.
-
Structural Basis for the Antiviral Activity of BST-2/Tetherin and Its Viral Antagonism.Front Microbiol. 2011 Dec 12;2:250. doi: 10.3389/fmicb.2011.00250. eCollection 2011. Front Microbiol. 2011. PMID: 22180752 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

